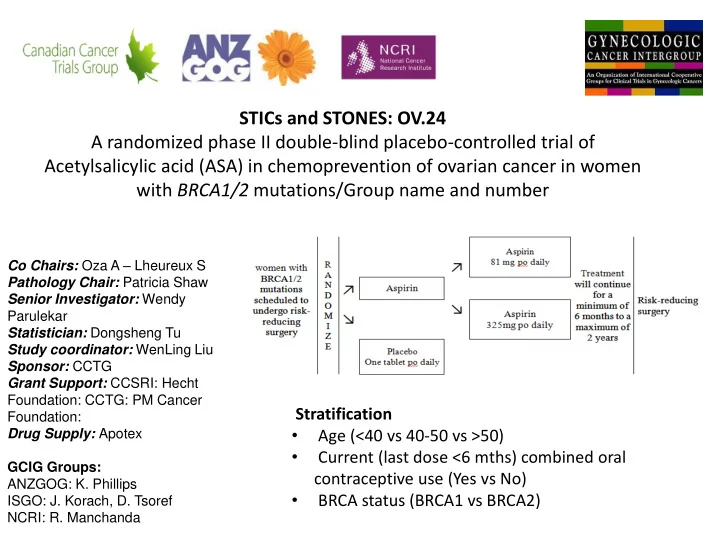

STICs and STONES: OV.24 A randomized phase II double-blind placebo-controlled trial of Acetylsalicylic acid (ASA) in chemoprevention of ovarian cancer in women with BRCA1/2 mutations/Group name and number Co Chairs: Oza A – Lheureux S Pathology Chair: Patricia Shaw Senior Investigator: Wendy Parulekar Statistician: Dongsheng Tu Study coordinator: WenLing Liu Sponsor: CCTG Grant Support: CCSRI: Hecht Foundation: CCTG: PM Cancer Stratification Foundation: • Drug Supply: Apotex Age (<40 vs 40-50 vs >50) • Current (last dose <6 mths) combined oral GCIG Groups: contraceptive use (Yes vs No) ANZGOG: K. Phillips • BRCA status (BRCA1 vs BRCA2) ISGO: J. Korach, D. Tsoref NCRI: R. Manchanda
Ongoing Trials – status update OV.24 / CCTG Trial setting: Women with germline BRCA1/2 mutations scheduled to undergo risk reducing surgery within 6 months to 2 years Study Design: Randomized 2:1, double-blind, placebo controlled phase II Sponsor(s): CCTG, ANZGOG Planned No. of patients: 414 Current accrual : Commencing Fall 2017 in Canada and Australia Other important information: Study has been submitted to Health Canada as pre-Clinical Trials Application. Pending further communication with regulatory authorities
A phase II, open-label, randomized, multi-centre study of neoadjuvant olaparib in ovarian cancer (NEO Trial) CT CT # CT CT� every� 9� weeks Biopsy* Biopsy* On� progression 6� cycles� platinum-based chemotherapy Platinum� Maintenance� R Olaparib Surgery sensitive� HGSOC Olaparib Olaparib ctDNA weekly ctDNA pre� each� cycle PIs: Oza AM & Brenton J. Sponsor: PMHC Grant Support: Investigator-initiated, AstraZeneca Global GCIG Groups: Belgium, Italy, New Zealand, and United Kingdom
Ongoing Trials – status update NEO / PMHC Trial setting: Platinum Sensitive Recurrent High Grade Serous Ovarian/Primary Peritoneal or Fallopian tube Cancer Study Design: Phase II, randomized, open-label Sponsor(s): PMHC Planned No. of patients: 75 Current accrual : 5 enrolled, 1 in screening Other important information: If positive signal, study will expand to N=150. Correlatives include: ctDNA; BRCA1/2 and HRD analysis; PARP expression; serial biopsy sites to examine heterogeneity of disease; at each biopsy time point ascitic fluid collection (where applicable).
Ongoing Trials – status update A proof of concept, multi-centre, clinical trial of the combination cediranib-olaparib at the time of disease progression on PARP inhibitor in ovarian cancer eVOLVE/PMHC Cediranib/Olaparib PARP inhibitor Previous therapy Orally daily >6 months Progression on PARP Proof of concept study If signal detected, trial continue to randomized phase 2 setting PIs: Oza AM Sponsor: PMHC Grant Support: Investigator-initiated, AstraZeneca Global
Ongoing Trials – status update eVOLVE/PMHC • A total of 30 patients with ovarian cancer who progressed on any PARP inhibitor that meet one of the following criteria: – Cohort of 10 platinum sensitive with no evidence of disease progression within 6 months of the last dose of platinum based chemotherapy and received PARP as their last line of treatment – Cohort of 10 platinum resistant with disease progression within 6 months of the last dose of a platinum based chemotherapy and received PARP inhibitor as their last line of treatment – Exploratory cohort of 10 patients who had progressed on PARP and then received standard chemotherapy with further disease progression
Ongoing Trials – status update eVOLVE/PMHC Trial setting: Recurrent high grade serous ovarian cancer Study Design: A multi centre, open-label, proof of concept study investigating the combination cediranib-olaparib at the time of disease progression on PARP inhibitor in ovarian cancer. Sponsor(s): PMHC Planned No. of patients: 31 Current accrual : 16 Other important information: Any prior PARP treatment allowed; platinum sensitive, resistant and exploratory cohorts; ctDNA analysis; archival tissue and up to 3 fresh core biopsies at the time of entry, and time of progression; germline BRCA1/2 ; PG analysis.
SUPPLEMENTAL SLIDES
Ongoing Trials – status update OV.24 / CCTG Stratification • Age (<40 vs 40-50 vs >50) • Current (last dose <6 mths) combined oral contraceptive use (Yes vs No) • BRCA status (BRCA1 vs BRCA2)
Ongoing Trials – status update OV.24 / CCTG • Secondary – To assess Patient Acceptance of Intervention of Chemoprevention Studies in this High-risk Female Cohort • Tertiary – To characterize the Effect of Aspirin on HGSOC Tumourigenesis • Link between Tumourigenesis and Microenvironment – Hormone stimulation – Inflammatory cell phenotype – Markers of inflammation and oncogenic pathway expression • Biobanking for future correlative studies
Recommend
More recommend