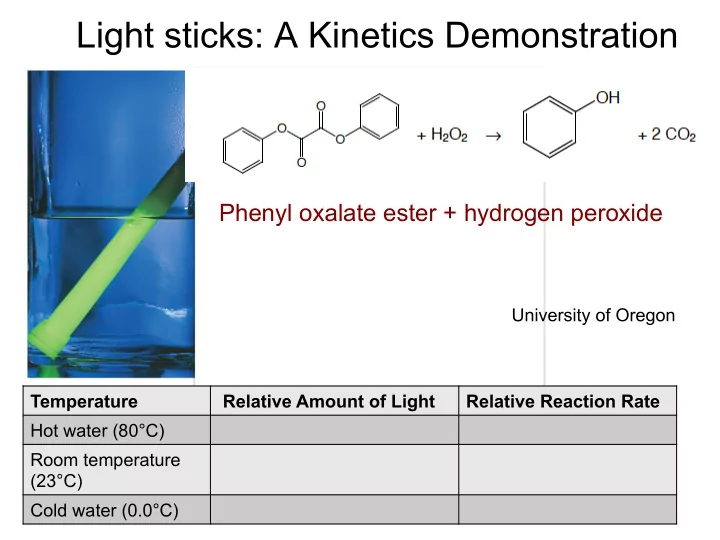

Light sticks: A Kinetics Demonstration Phenyl oxalate ester + hydrogen peroxide University of Oregon Temperature Relative Amount of Light Relative Reaction Rate Hot water (80°C) Room temperature (23°C) Cold water (0.0°C)
Light sticks: A Kinetics Demonstration Temperature Relative Amount of Light Relative Reaction Rate Hot water (80°C) Bright Room temperature Medium (23°C) Cold water (0.0°C) Dim
Temperature and Rate • Generally, as temperature increases, so does the reaction rate. • This is, in part, because k is temperature-dependent.
Light sticks: A Kinetics Demonstration Temperature Relative Amount of Light Relative Reaction Rate Hot water (80°C) Bright Fast Room temperature Medium Medium (23°C) Cold water (0.0°C) Dim Slow
Temperature effects: Consider the humble light stick. light stick mechanism When the temperature increases, the molecular speed __________ . increases increases When the molecular speed increases, the number of collisions ________ . Therefore, the rate of reaction __________ when the temperature increases (always) increases. However, there is more to it than that.
Gas kinetic rate: • At STP, there are 10 10 collisions per second. • This sets the upper limit for reaction rates. Why aren’t all reactions over within a fraction of a second? Apparently, not all collisions are effective at leading to a chemical reaction.
Summary of Light Stick Reaction Mechanism
Maxwell–Boltzmann Distributions • Temperature is T 2 > T 1 defined as a measure of the Minimum average kinetic energy needed to energy of the overcome E a molecules in a sample. A larger fraction of molecules reacts at a higher temperature. • At any temperature there is a wide distribution of molecular speeds of the molecules. There is a distribution of kinetic energies of the molecules.
Maxwell–Boltzmann Distributions T 2 > T 1 Minimum energy needed to overcome E a A larger fraction of molecules reacts at a higher temperature. • As the temperature increases, the curve broadens. • At higher temperatures, a larger population of molecules has a higher energy and therefore can react.
At a higher temperature, the fraction of collisions with sufficient energy equal to or greater than E a increases. Reaction rate therefore increases. The increase in kinetic energy is the primary reason that reaction rates increase at higher temperatures.
Recommend
More recommend