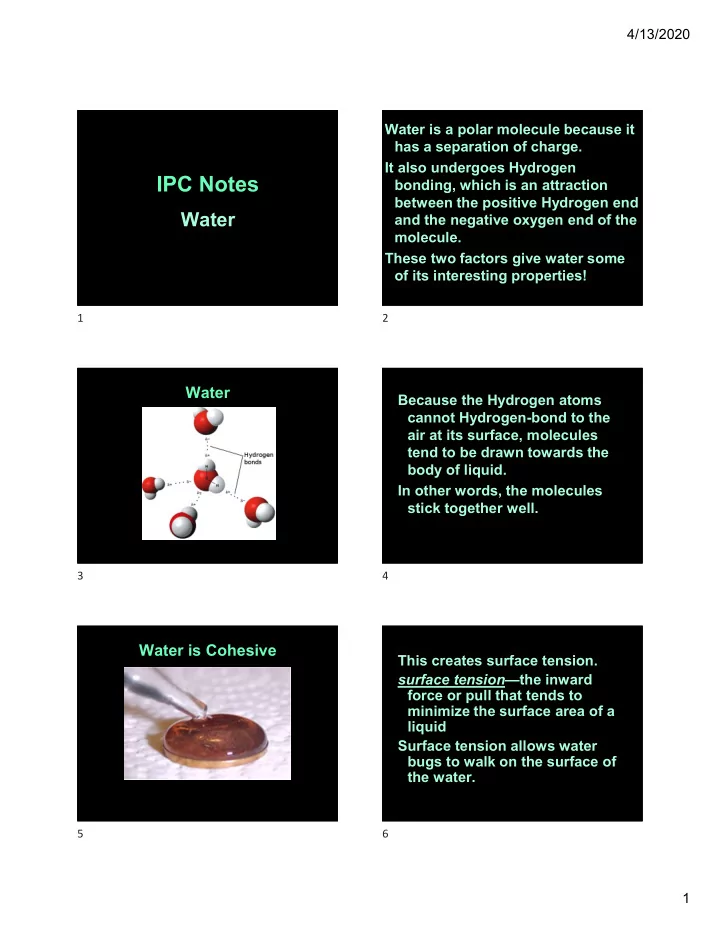

4/13/2020 Water is a polar molecule because it has a separation of charge. It also undergoes Hydrogen IPC Notes bonding, which is an attraction between the positive Hydrogen end Water and the negative oxygen end of the molecule. These two factors give water some of its interesting properties! 1 2 Water Because the Hydrogen atoms cannot Hydrogen-bond to the air at its surface, molecules tend to be drawn towards the body of liquid. In other words, the molecules stick together well. 3 4 Water is Cohesive This creates surface tension. surface tension —the inward force or pull that tends to minimize the surface area of a liquid Surface tension allows water bugs to walk on the surface of the water. 5 6 1
4/13/2020 Surface Tension A surfactant is a substance that decreases surface tension of a liquid. In water, it does this by interfering with the hydrogen bonding. Detergent is a good example of a surfactant. 7 8 When water freezes, unlike most Water boils and freezes at much other substances, it becomes higher temperatures than most less dense and it floats! other substances that have When water freezes, it forms a similar molar masses. Why? very open crystalline structure. Water molecules are held together by very strong hydrogen bonding. 9 10 It is very important for life on Which phase of water has the earth that water floats when it lowest density? freezes. Which phase has the highest An insulating layer of ice in the density? winter keeps the oceans from freezing and killing everything. 11 12 2
4/13/2020 The negative side of water Water is called the universal molecules attract the positive solvent because so many side of a polar molecule. different substances will The same thing happens with the dissolve in it. negative side. 13 14 Electrolytes are any ionic Nonpolar substances are able to compound that will conduct dissolve other nonpolar electricity when dissolved in substances, due to the lack of water. repulsive forces. Non-electrolytes—like sugar— This leads to the saying “like will dissolve but do not conduct dissolves like.” electricity. 15 16 A colloid is a mixture with Mixtures where particles settle particles intermediate in size out upon standing are called between a solution and a suspensions. Suspensions suspension. have the largest particles! They differ from solutions (which are homogeneous) because their particles are much larger. 17 18 3
4/13/2020 Colloid A colloid can be discerned due to the Tyndall effect . Light scatters as it shines through a colloid. You will see both light and particles. Fog is a good example of a colloid. You don’t want to use your high beam lights when it is foggy outside. 19 20 4
Recommend
More recommend