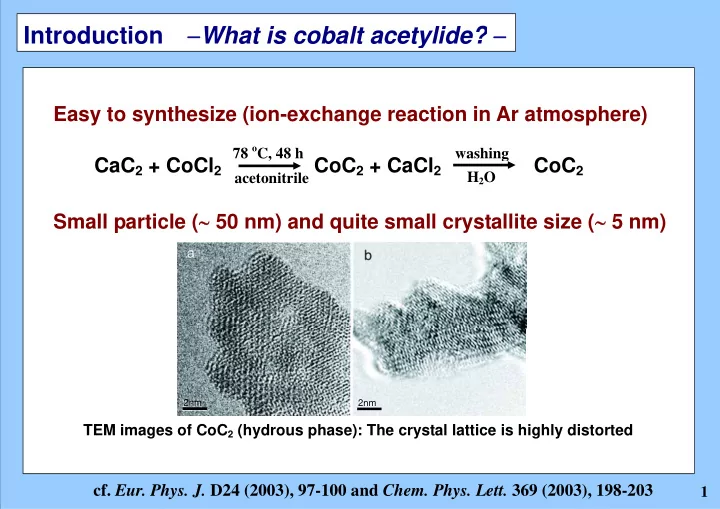

Introduction − What is cobalt acetylide? − Easy to synthesize (ion-exchange reaction in Ar atmosphere) 78 o C, 48 h washing CaC 2 + CoCl 2 CoC 2 + CaCl 2 CoC 2 H 2 O acetonitrile Small particle ( ∼ 50 nm) and quite small crystallite size ( ∼ 5 nm) 2nm 2nm TEM images of CoC 2 (hydrous phase): The crystal lattice is highly distorted cf. Eur. Phys. J. D24 (2003), 97-100 and Chem. Phys. Lett. 369 (2003), 198-203 1
Rod-shape large crystallite particles can be synthesized at 100 o C Ferromagnetism of smaller particles The particle shows ferromagnetism even in room temperature! 2
Question: What's the role of the absorbed water? CoC 2 ··· Highly absorbent of water (ca. 1-2 H 2 O / Co 2+ ) CoC 2 is small, therefore such a large amount of the absorbed water must changes the crystal structure and the physical properties of CoC 2 . Here, we reveal the effect of the absorbed water Sample preparation Anhydrous CoC 2 : CaC 2 (5 mmol) and CoCl 2 (5.2mmol) in 300 ml of acetonitrile was heated at 78 o C, 130 h in grove-box. *Anhydrous CoC 2 contains ca. 35 % of CaC 2 Hydrous CoC 2 : Anhydrous CoC 2 was exposed to air (25 o C, humidity 70 %) or washed with water (for XRD to remove CaC 2 peaks). 3
Crystal structure of the anhydrous CoC 2 XRD result of anhydrous CoC 2 4.82 Å 3 CoC 2 (calc) Intensity / 10 4 counts CaC 2 (calc) exp. 3.41 Å 2 3.41 Å Anhydrous CoC 2 : Cubic 2 − → Orientation disorder of C 2 1 Sharp peaks → Large domain Synchrotron radiation, 0.9988 Å 0 Crystallite size > 45 nm 3 2.5 2 1.5 1 (Single domain particle) d -value / Å 4
Crystal structure of the hydrous CoC 2 EXAFS result Structural model for calculated spectrum (MgC 2 -type structure) ·Tetragonal lattice 2 − dianions are ordered ·C 2 2 − − Co 2+ chain || c -axis ·Co 2+ − C 2 5
XRD result of hydrous CoC 2 3.35 Å 6 3.78 Å Intensity / 10 2 counts exp. 3.78 Å calc. (based on EXAFS) 4 EXAFS spectrum is 2 consistent with XRD pattern Cu K α , 1.5418 Å Broad peaks → small domain 0 3 2.5 2 1.5 1 Crystallite size: ∼ 10 nm Å d -value / (consistent with TEM images) 6
Susceptibility of the anhydrous and hydrous CoC 2 15 5 ZFC, H = 10 Oe FC, H = 10 Oe 4 anhydrous χ / 10 -3 emu g -1 χ / 10 -3 emu g -1 anhydrous air 10 min 10 air 10 min air 30 min 3 air 30 min air 60 min air 60 min 2 5 1 0 0 0 10 20 30 40 0 10 20 30 40 T / K T / K *FC: cooled with H = 10 Oe Anhydrous CoC 2 : Paramagnetic Air-exposure induce the ferromagnetism 7
Magnetization curves of the anhydrous and hydrous CoC 2 20 6 anhydrous anhydrous 10 min 10 min 4 20 min 30 min 10 30 min 60 min M / emu g -1 2 M / emu g -1 0 0 -2 -10 1.8 K -4 1.8 K -20 -6 -1 -0.5 0 0.5 1 -0.1 0 0.1 H / T H / T Coercive force and remanent magnetization are raised by water absorption 8
Mechanism of the water-induced ferromagnetism 2 − − Co 2+ configurations Possible Co 2+ − C 2 Ferromagnetic interaction Perpendicula Intermediate Parallel Hydrous CoC 2 (ferromagnetic) Anhydrous CoC 2 Weak interaction (superparamagnetic) (FM or AFM) 9
Schematic model of the influence of water absorption Water absorption Small superparamagnetic clusters Expanded by water 2 − Large ferromagnetic domain C 2 Ferromagnetic configuration Ferromagnetism! (strong) Co 2+ 10
Summary Anhydrous CoC2 Hydrous CoC 2 Lattice: Cubic Tetragonal 2 − : Disordered Orientation of C 2 Ordered Magnetism: Superparamagnet Ferromagnet Structural domain: Large ( ∼ 50 nm) Small ( ∼ 10 nm) 2 − Water-absorption induce the orientation ordering of C 2 Water-induced ferromagnetism 11
The other feature of MC 2 compound − easy synthesis of nanoalloy − Heating the MC 2 compounds gives "carbon-coated metal nanoparticle" Δ Δ ∼ 250 o C ∼ 250 o C Carbon-coated metal MC 2 MC 2 + C + metal (5 - 20 nm) 20 nm cf. Co@C cf. Fe@C cf. Appl. Phys. Lett. 84 (2004), 1753-1755 12
If the mixture of MCl 2 and M'Cl 2 is used for synthesis, nanoalloy can be easily obtained only by heating! Carbon-coated alloy M x M' 1-x C 2 FeCo@C NiPd@C Fe:Co ∼ 1:1 Ni:Pd ∼ 1:1 13
Recommend
More recommend