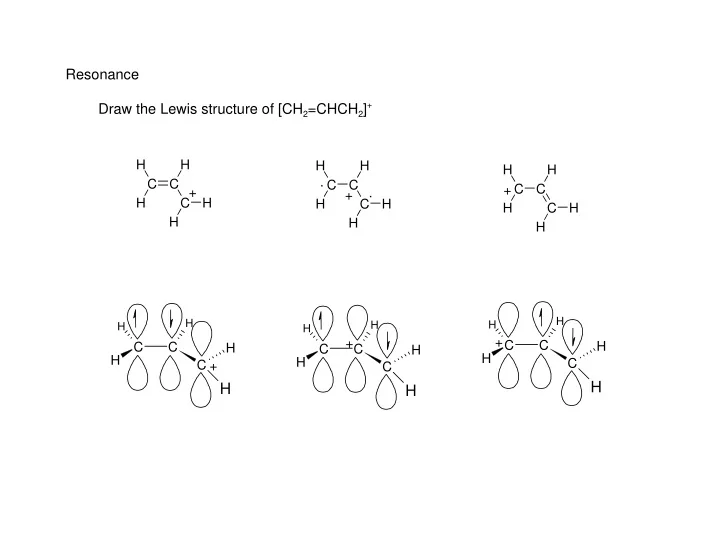

Resonance Draw the Lewis structure of [CH 2 =CHCH 2 ] + H H H H H H . C C C C C C + + . + H C H H C H H C H H H H H H H H H H + + C C H C C H C C H H H H C C + C H H H
Resonance Draw the Lewis structure for nitromethane, CH 3 NO 2 - . . .. . H . . H O . O . . C N C N + + H H .. . .. O O . . . H . . H -
H1s 2 Osp 3 Csp H1s 2 Nsp .. .. H O + 2 Osp H1s C N H : O : H .. H1s .. H : O : + C N H 2 Osp . O . H .. 3 Csp H1s 2 Nsp 2 Osp H1s
Common structural classes where resonance is observed Compounds with allylic lone pairs .. .. .. - : : O O .. -
Common structural classes where resonance is observed Compounds with allylic positive charge + + +
Common structural classes where resonance is observed Compounds with lone pairs next to positive charge .. . . + Br : Br .. .. +
Nitromethane, CH 3 NO 2 , combines two structural classes where resonance is observed It is a compound with lone pairs next to positive charge and allylic lone pairs - . . .. . H . . H O . O . . C N C N + + H H .. . .. O O . . . H . . H -
Common structural classes where resonance is observed Compounds with pi bonds between atoms of different electronegativities . .. .. - . O : : O +
Common structural classes where resonance is observed Compounds with aromatic rings H H H H H H
The concepts of resonance 1. Resonance is not something that is happening to a molecule. It is a way to describe the electronic distribution of a molecule. 2. Individual resonance isomers are imaginary, not real. a. They are representations of a hybrid structure b. The resonance hybrid is more stable than any individual resonance isomer contributing to it 3. Resonance isomers must be valid Lewis structures. 4. Resonance isomers differ only by the relative placement of their pi and nonbonding electrons in p orbitals. 5. Resonance isomers must have identical constitutions. Therefore, the hybridization states of the constituent atoms cannot change. 6. Resonance isomers must have the same number valence electrons and the same number of paired and unpaired electrons. 7. Different resonance isomers don’t have to be equivalent. Resonance isomers that describe the electronic configuration of a molecule most realistically have a. all octets full b. minimal formal charge c. minimal formal charge separation d. formal charge assignment according to relative electronegativities
Heterocyclic aromatic compounds : pyridine H H H H H H H H H .- . X H N H H N H H N H .. .. + H H H N H H
Consider resonance isomers of spinacine .. . . O H H .. N - : + O .. H . N . N . .
Recommend
More recommend