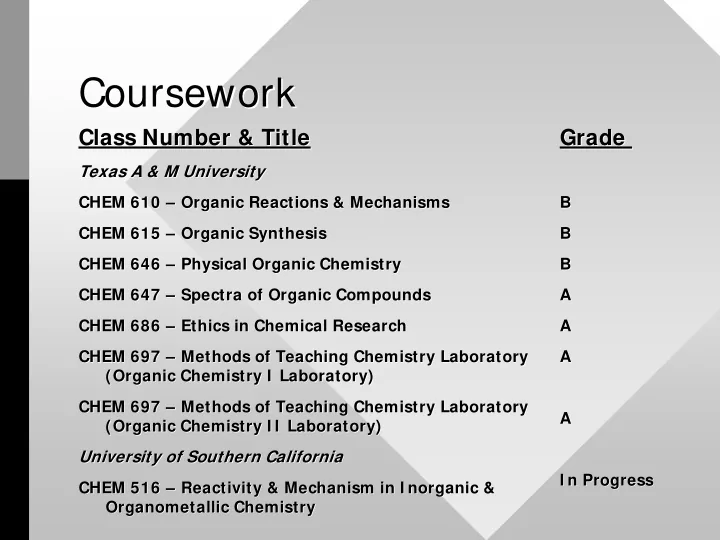

Coursework Coursework Class Number & Title Grade Class Number & Title Grade Texas A & M University Texas A & M University CHEM 610 – – Organic Reactions & Mechanisms Organic Reactions & Mechanisms B CHEM 610 B CHEM 615 CHEM 615 – – Organic Synthesis Organic Synthesis B B CHEM 646 – – Physical Organic Chemistry Physical Organic Chemistry B CHEM 646 B CHEM 647 – – Spectra of Organic Compounds Spectra of Organic Compounds A CHEM 647 A CHEM 686 – – Ethics in Chemical Research Ethics in Chemical Research A CHEM 686 A CHEM 697 – CHEM 697 – Methods of Teaching Chemistry Laboratory Methods of Teaching Chemistry Laboratory A A (Organic Chemistry I Laboratory) (Organic Chemistry I Laboratory) CHEM 697 CHEM 697 – – Methods of Teaching Chemistry Laboratory Methods of Teaching Chemistry Laboratory A A (Organic Chemistry I I Laboratory) (Organic Chemistry I I Laboratory) University of Southern California University of Southern California I n Progress I n Progress CHEM 516 – – Reactivity & Mechanism in I norganic & Reactivity & Mechanism in I norganic & CHEM 516 Organometallic Chemistry Organometallic Chemistry
Electron Transport Materials Electron Transport Materials Based on Cyclodiborazane Based on Cyclodiborazane Sean Owen Clancy Sean Owen Clancy Advisor: Aaron W. Harper Advisor: Aaron W. Harper 8 February 2001
Outline Outline • History • History • Background • Background • Synthesis • Synthesis • Characterization • Characterization • Applications • Applications
History History • MacDiarmid, Heeger, and Shirakawa (1977) – – • MacDiarmid, Heeger, and Shirakawa (1977) Electrically Conductive Polymers Electrically Conductive Polymers • Burroughes et al. (1990) – – Electroluminescence (EL) in Electroluminescence (EL) in • Burroughes et al. (1990) Conjugated Polymers Conjugated Polymers π - Development of purportedly π • Chujo et al. (1992) – – Development of purportedly - • Chujo et al. (1992) π - type π electron- -deficient n deficient n- -type -conjugated conjugated electron polycyclodiborazane polycyclodiborazane
Background Background • Advantages / Disadvantages of PLEDs versus • Advantages / Disadvantages of PLEDs versus Conventional Inorganic LEDs Conventional Inorganic LEDs • Charge Transport • Charge Transport • Limitations of Electron Transport Materials • Limitations of Electron Transport Materials • Cyclodiborazane • Cyclodiborazane • Single- -Layer Device Layer Device • Single • Triple- -Layer Device Layer Device • Triple
Advantages/Disadvantages of Advantages/Disadvantages of PLEDs vs. Inorganic LEDs PLEDs vs. Inorganic LEDs Advantages Disadvantages Advantages Disadvantages • Cost • Lifetime • Cost • Lifetime • Stability • • Flexible substrate Stability • Flexible substrate • Size • Phase segregation • Size • Phase segregation
Charge Transport Charge Transport – Background: Background: – • The band gap is a region where no formal energy • The band gap is a region where no formal energy levels reside. levels reside. • All states below the gap are occupied and form the • All states below the gap are occupied and form the π band,” a.k.a. the valence band. “ π band,” a.k.a. the valence band. “ π * States above the band are empty and form the “ π • * • States above the band are empty and form the “ band,” a.k.a. the conduction band. band,” a.k.a. the conduction band. π band” is called the HOMO. The top of the “ π • band” is called the HOMO. • The top of the “ π * band” is called the LUMO. The bottom of the “ π • * band” is called the LUMO. • The bottom of the “
Charge Transport Charge Transport Structure of a Single- -Layer Device: Layer Device: Structure of a Single High work function metal electrode: e.g. Al or Ca Low work Bipolar Charge Transport Layer with function Indium Doped Lumophore Tin Oxide (ITO) electrode on Glass Structure of a Triple- -Layer Device: Layer Device: Structure of a Triple High work function metal electrode: e.g. Al or Ca Emitting or Photocharge Electron-Transport Layer Generating Layer Hole-Transport Layer Low work function Indium Tin Oxide (ITO) electrode on Glass
Charge Transport Charge Transport • When voltage is applied, a radical anion is formed in • When voltage is applied, a radical anion is formed in the ETL when the HWFM injects an electron into the the ETL when the HWFM injects an electron into the ETL. ETL. π - This radical anion species travels through the π • - • This radical anion species travels through the conjugation of the ETL towards the positive electrode conjugation of the ETL towards the positive electrode / LWFM. / LWFM. • A radical cation is formed in the HTL when the LWFM • A radical cation is formed in the HTL when the LWFM removes an electron from the HTL. removes an electron from the HTL. π - This radical cation species travels through the π • - • This radical cation species travels through the conjugation of the HTL towards the negative electrode conjugation of the HTL towards the negative electrode / HWFM. / HWFM.
Charge Transport Charge Transport • Charge recombination and formation of exciton. • Charge recombination and formation of exciton. • Formation of singlet and triplet excited states. • Formation of singlet and triplet excited states. • A photon is released when the singlet state relaxes. • A photon is released when the singlet state relaxes.
Charge Transport Charge Transport – Single Single- -Layer Device: Layer Device: – h ν HWFM LWFM Legend: Legend: HWFM = High Work Function Metal, e.g. Al or Ca HWFM = High Work Function Metal, e.g. Al or Ca ETL = Electron Transport Layer ETL = Electron Transport Layer EML = Emissive Layer EML = Emissive Layer HTL = Hole Transport Layer HTL = Hole Transport Layer LWFM = Low Work Function Metal, e.g. ITO (Indium- -Tin Oxide) Tin Oxide) LWFM = Low Work Function Metal, e.g. ITO (Indium
Charge Transport Charge Transport – Triple Triple- -Layer Device: Layer Device: – HWFM h ν EML EML LWFM HTL ETL Legend: Legend: HWFM = High Work Function Metal, e.g. Al or Ca HWFM = High Work Function Metal, e.g. Al or Ca ETL = Electron Transport Layer ETL = Electron Transport Layer EML = Emissive Layer EML = Emissive Layer HTL = Hole Transport Layer HTL = Hole Transport Layer LWFM = Low Work Function Metal, e.g. ITO (Indium- -Tin Oxide) Tin Oxide) LWFM = Low Work Function Metal, e.g. ITO (Indium
Other Electron Transport Other Electron Transport Materials Materials Material Deficiencies Material Deficiencies Tris(8- -hydroxyquinolinato)aluminum hydroxyquinolinato)aluminum Tris(8 • • Presence of charge traps. Presence of charge traps. N O O • Quenching of EL by • Quenching of EL by Al crystallization of amorphous crystallization of amorphous N N O Alq3. Alq3. Alq 3 Alq 3 Oxadiazoles Oxadiazoles • • Can undergo irreversible redox Can undergo irreversible redox reactions. reactions. O N PBD PBD N
Other Electron Transport Other Electron Transport Materials Materials Material Deficiencies Material Deficiencies Thiophenes Thiophenes • Electronic properties are • Electronic properties are temperature dependent. temperature dependent. N N C(CH 3 ) 3 (H 3 C) 3 C S O O BBOT BBOT PPVs with electron- -withdrawing groups withdrawing groups PPVs with electron MeO • Conjugation shortened to make • Conjugation shortened to make O(CH 2 ) 10 O CN OMe polymer easier to process. polymer easier to process. MeO NC n • • Chemically reactive. Chemically reactive. OMe PC10 PC10
Cyclodiborazane Cyclodiborazane π - Contains electron deficient boron in the π • -system. system. • Contains electron deficient boron in the • Boron in conjugation delocalizes electron deficiency • Boron in conjugation delocalizes electron deficiency rather than providing an electron sink/trap. rather than providing an electron sink/trap. • Semi- -empirical calculations (AM1 level, Spartan MOPAC empirical calculations (AM1 level, Spartan MOPAC • Semi program) of a model compound showed a LUMO that program) of a model compound showed a LUMO that suggests that the electron deficiency is effectively suggests that the electron deficiency is effectively delocalized. delocalized. • Lobes at ends of the model suggest that conjugation • Lobes at ends of the model suggest that conjugation can be extended. can be extended.
Recommend
More recommend