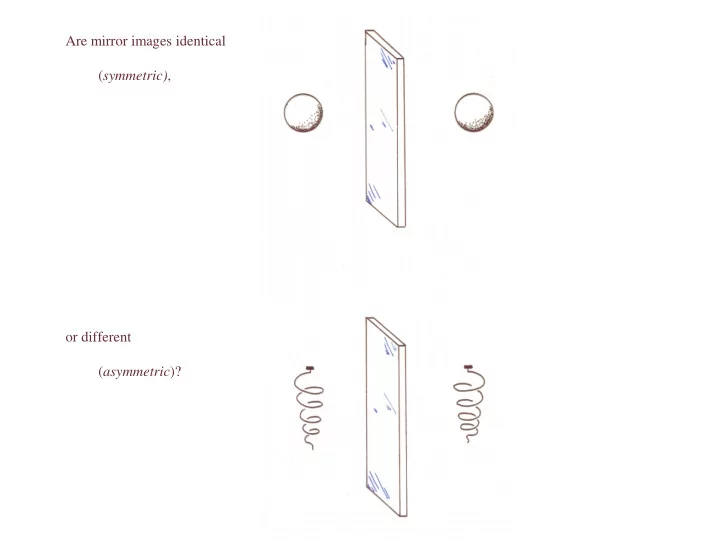

Are mirror images identical ( symmetric) , or different ( asymmetric )?
Left hand mirror Right hand the left and right hands are nonsuperimposable
A molecular case: the amino acid alanine Symmetric or asymmetric?
Molecules with the same constitution that differ only by the relative positions of their atoms in space: � stereoisomers! Molecular stereoisomers that are nonsuperimposable mirror images are called enantiomers � An enantiomer possesses the property of molecular chirality . • Enantiomers Usually have 4 different functional groups bonded to an sp 3 -hybridized atom. That atom is • referred to as a � Stereogenic center � Stereocenter � Chiral center • Have identical physical properites save for one: they cause exactly opposite rotations of plane-polarized light and so are said to exhibit optical activity . � A clockwise rotation is d � � � (+) dextrorotatory � A counterclockwise rotation is l � � � (-) levorotatory
Light: electromagnetic radiation Electric field Electric wave Magnetic Magnetic wave field Oscillating orthogonal electric and magnetic fields of a beam of light
Plane-polarized light and its consequences 1st polarizing 2nd polarizing filter filter observer sees maximum light source plane- polarized light observer sees no light plane- polarized light
Polarimeter: An instrument to measure optical activity
Defining a physical constant: Specific rotation [ α ] � C D = 100 α cl � = the observed rotation of plane-polarized light in degrees c = the concentration in g / 100mL l = the pathlength (always 1 dm) Enantiomeric purity ( % e.e. ): % e.e. = � [(+)-enantiomer] - [(-)-enantiomer] � x 100 [(+)-enantiomer] + [(-)-enantiomer] % e.e. = α x 100 [ α ] � C D Note: a 50 : 50 mixture of enantiomers has no � . It appears to be achiral . Such mixtures are called racemates .
SO WHAT? Is molecular chiralty important? Consider: • Gloves • Smells • Drug-receptor interactions Different stereoisomers like enantiomers interact with one another in different ways!
Stereochemical theory of odor
H H O O ( S )-(+)-CARVONE ( R )-(-)-CARVONE smells like caraway seeds smells like spearmint gum H H ( R )-(+)-LIMONENE ( S )-(-)-LIMONENE smells like citrus fruit smells like turpentine
Some drugs that exist as enantiomers and why this matters OH H O O O (-)-ibuprofen (+)-ibuprofen [ α ] D 25 = - 20.6 � (c 1.00, ethanol) [ α ] D 25 = + 58.5 � (c 2.00, ethanol) Advil, Motrin OTC for arthritis, fever; general anti-inflammatory analgesic (+)-enantiomer is the active isomer (-)-enantiomer is inactive the enzyme 2-arylpropionyl-CoA epimerase converts (-)-enantiomer to the (+)-enantiomer (Zhao, X.; Eur. J. Med. Chem . 2006 , 41 , 1352-1358; Piccolo, O.; J. Org. Chem . 1985 , 50 , 3945-3946.)
Some drugs that exist as enantiomers and why this matters O O O O N N N H H N O O O O ( - )-thalidomide ( + )-thalidomide 25 = - 64.6 � (DMF) 25 = + 64 � (DMF) [ α ] D [ α ] D teratogen antiemetic enantiomers are interconvertable (undergo racemization) in vivo racemate is immunomodulator used to treat erythema nodosum leprosum (in combination with dexamethasone) newly diagnosed multiple myeloma actinic prurigo (Ellis, G.P.; West, G.B. Progress in Medicinal Chemistry , Elsevier: New York, 198, p 170)
Some drugs that exist as enantiomers and why this matters H H N N Cl Cl O O ( - )-ketamine ( + )-ketamine [ α ] D 25 = - 91.88 � (c 2.00, H 2 O) [ α ] D 25 = + 92.48 � (c 2.00, H 2 O) analgesic racemate used to induce general anesthesia ( + )-enantiomer (Ketanest S) is more potent ( - )-enantiomer is more hallucinogenic: may cause nightmares (Marrietta, M. P.; Way, W.L.; Castagnoli, N. Jr.; Trevor, A.J. J. Pharmacol. Exp. Ther . 1977 , 202 , 157-165.)
The Alan Baxter story 2002 Salt Lake City Winter Olympics bronze medalist in downhill skiing (slalom) failed IOC routine drug test: + for methamphetamine source of + : Vicks nasal spray U.S. version contains the legal enantiomer of methamphetamine: H H N N (+)-methamphetamine (-)-methamphetamine (decongestant) (crank) IOC test did not differentiate between enantiomers Baxter cleared, but lost his medal – now IOC explicitly bans both enantiomers 2006 Turin Winter Olympics Baxter finishes 16 th
How do constituional isomers differ from stereoisomers? What kinds of stereoisomers are there?
What ways are there to draw enantiomers? 1. Hatched and wedged line projections (most popular) H CH 3 H 3 C H are these these enantiomers, or just different hatched and wedged line projections of the same molecule?
Remember carvone and limonene? Do these have R or S configurations? H H O O H H
What ways are there to draw enantiomers? 3. Newman projections CH 2 CH 3 CH 2 CH 3 H CH 3 CH 2 H H H 3 C H H CH 3 CH 2 CH 3 H Are these anti-staggered and gauche staggered conformations of the same molecule?
1 What ways are there to draw enantiomers? CH 3 4. Fisher projections 2 C H 3 H 2 C Br \ H 3 C 4 body lines up with the parent chain of the molecule and your a. Orient your point of view so that your line of sight bisects the bond angle formed by the remaining two substituents of the chiral center . b. Draw the molecule as it appears to you CH 3 using wedged and hatched lines: Br C H CH 2 CH 3
c. The Fisher convention replaces wedged and hatched lines with regular ones. • The vertical lines of a chiral center are always pointing away from you • The horizontal lines of a chiral center are always pointing towards you CH 3 CH 3 Br H Br C H becomes CH 2 CH 3 CH 2 CH 3 CH 2 CH 3 CH 2 CH 3 CH 2 CH 3 H O H H OH CH 3 CH 2 CH 2 CH 2 OH CH 2 CH 2 CH 2 CH 3 CH 2 CH 2 CH 2 CH 3 H Two of these Fisher projections of different conformations of the same configuration. Which two?
Sterochemical outcomes of reaction involving 1 stereogenic center: 1. optically inactive starting materials + optically inactive reagents = optically inactive products Br HBr (racemic) 0 � C, dark * Br Br 2 * H 2 O (racemic) OH 2. optically active starting materials with spectator stereocenter + optically inactive reagents = optically active products H H H 3 C H 3 C H 2 Pd /C ( S ) ( S )
3. optically active starting materials with participating stereocenter + optically inactive reagents = products of the same configuration: Retention • products of the opposite configuration: Inversion • a mixture of retention and inversion products: Racemization • Br H H 3 C H 3 C Br 2 Cl Cl * light (racemic) why? A symmetrical ( achiral ) intermediate forms: Cl H 3 C
A molecule with 2 chiral centers and 4 stereoisomers 2,3-dihydroxybutanoic acid, also called tartaric acid: OH O * * OH OH • Draw all four R , S isomers of this molecule Note: the maximum number of nonconformational stereoisomers a moleclue can have is = 2 n where n = the number of stereogenic “parts” of the molecule = number of chiral centers + number of E,Z double bonds
A molecule with 2 chiral centers and 4 stereoisomers O O CO 2 H CO 2 H H H H O OH OH H OH H O H H O H OH H O H H enantiomers H CH 3 H 3 C H O OH CH 3 CH 3 (2 R ,3 R ) (2 S ,3 S ) diastereomers O O CO 2 H H H CO 2 H H O OH H OH OH H O H O H H O H H O H OH OH CH 3 H 3 C enantiomers CH 3 H H CH 3 (2 R ,3 S ) (2 S ,3 R )
A molecule with 2 chiral centers and 3 stereisomers: one isomer is a meso isomer OH OH Consider with 2 chiral centers and (not counting conformational ismoers) 2 2 = 4 possible stereoisomers, but there are only 3! CH 3 CH 3 H H CH 3 H 3 C H O OH H OH H O H H OH H O H H H the same CH 3 H 3 C H O OH CH 3 CH 3 (2 S ,3 R ) = meso (2 R ,3 S ) = meso diastereomers diastereomers CH 3 H H CH 3 CH 3 H 3 C H O OH H OH H O H H O H H O OH H OH CH 3 H 3 C enantiomers CH 3 H H CH 3 (2 S ,3 S ) (2 R ,3 R )
Stereochemical outcomes of reactions involving 2 or more stereogenic centers: 1. Anti-addtion of X 2 to alkenes: diastereoselective Br H Br H H Br 2 CH 3 H H 3 C + H 3 C H 3 C CH 3 H H CCl 4 Br CH 3 Br ( Z ) (2 R , 3 R ) (2 S , 2 S ) CH 3 CH 3 H Br Br H + Br H H Br CH 3 CH 3 threo -2,3-dibromobutane (a racemate)
Br H Br H 3 C CH 3 Br 2 CH 3 H 3 C CH 3 + H H 3 C H H H CCl 4 Br H Br ( E ) (2 S , 3 R ) (2 R , 2 S ) the same! CH 3 H Br H Br CH 3 erythro -2,3-dibromobutane (meso) • All anti-additions behave in a similar, diastereoselective manner.
• Syn-addition of H 2 to alkenes: diastereoselective H H H 2 Pt H ( S ) (1 S , 2 S ) 1 enantiomer H H 2 H + Pt H H ( Z ) (3 R , 4 S ) (3 S , 4 R ) racemate • All syn-additions behave in a similar diastereoselective manner.
Recommend
More recommend