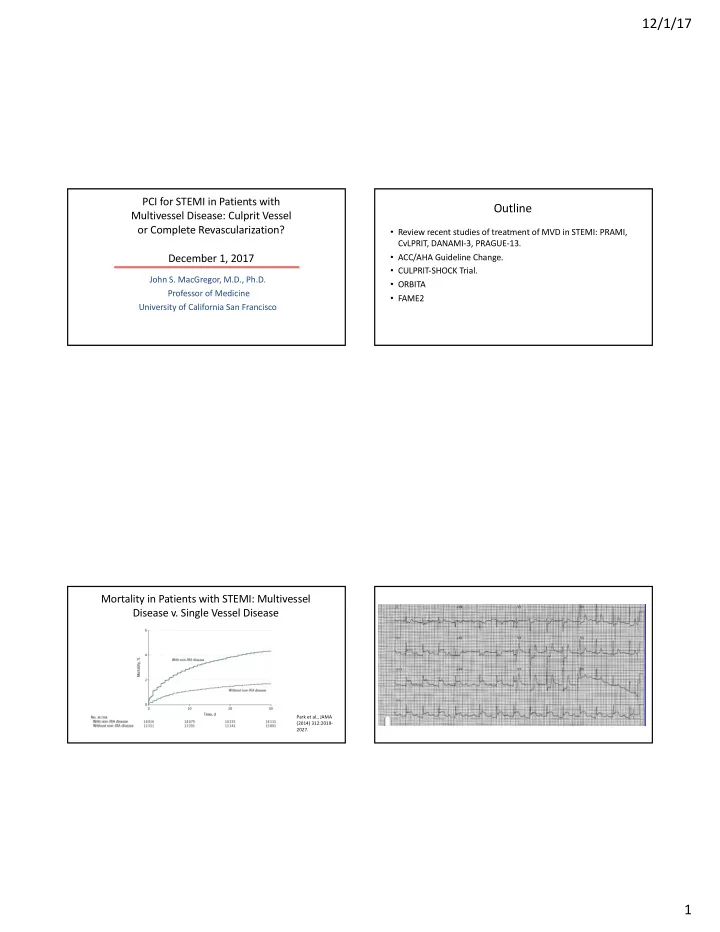

12/1/17 PCI for STEMI in Patients with Outline Multivessel Disease: Culprit Vessel or Complete Revascularization? • Review recent studies of treatment of MVD in STEMI: PRAMI, CvLPRIT, DANAMI-3, PRAGUE-13. December 1, 2017 • ACC/AHA Guideline Change. • CULPRIT-SHOCK Trial. John S. MacGregor, M.D., Ph.D. • ORBITA Professor of Medicine • FAME2 University of California San Francisco Mortality in Patients with STEMI: Multivessel Disease v. Single Vessel Disease Park et al., JAMA (2014) 312:2019- 2027. 1
12/1/17 Potential Strategies Aggressive Approach: Acute treatment of all angiographically significant lesions. Conservative Approach: Acute treatment of only the infarct- related artery, with medical therapy for the other lesions unless ischemia occurs. In Between: Acute treatment of only the infarct-related artery, with staged treatment of the other lesions. 2
12/1/17 Acute Multivessel PCI During STEMI Treat Infarct-Related Artery Only Widimsky and Holmes Eur. Hrt. J. 2011, Widimsky and Holmes Eur. Hrt. J. 32:396-403 2011, 32:396-403 Acute Treatment of Infarct-Related Artery with Practice Variability in the Use of Multivessel PCI in Staged Treatment of Other Lesions Primary PCI for STEMI Widimsky and Holmes Eur. Hrt. J. 2011, Cavender et al., 32:396-403 AJC (2009) 104:507-513. 3
12/1/17 ACC/AHA Guidelines for MV-PCI in Patients with ACC/AHA Guidelines: Class of Recommendation MVD and STEMI: 2013 v. 2015 Recommendation JACC (2016)67:1235- JACC (2016) 1250. 67:1235-1250. Acute MV-PCI v. Staged MV-PCI for MVD in STEMI: Meta-Analysis of Studies Comparing Acute MV-PCI One Year Mortality Outcomes - HORIZONS-AMI to Staged PCI for Mortality in Patients with STEMI Vlaar et al., JACC Kornowski et al., JACC (2011) (2011) 58:692-703. 58:704-11. 4
12/1/17 Kaplan-Meier Curves for Primary Outcome in the PRAMI PRAMI Study 465 patients with STEMI and MVD. Randomized to IRA PCI only or IRA plus MV-PCI. End point: cardiac death, non-fatal MI, refractory angina. Wald et al., NEJM (2013) 369:1115- 1123. Event Rate: IRA Only v. MV-PCI in DANAMI-3- DANAMI-3-PRIMULTI PRIMULTI 627 patients with STEMI and MVD. After successful treatment of the IRA, randomized to no further treatment (313) or complete FFR guided revascularization (314). Primary end point: death, non-fatal MI, ischemia driven revascularization. Engstrom et al., Lancet (2015) 386:665-71. 5
12/1/17 Recent Trials of Complete v. Culprit Lesion Only Meta-Analysis: Cardiovascular Mortality – Revascularization in Patients with STEMI Multivessel PCI v. Culprit Only Villablanca et al., Int. J. Cardiol. (2016) Binder et al., Eur. Hrt. J.( 2016) 220:251-259. 37:217-220. Mortality at 30 Days: Multivessel PCI v. Culprit Culprit-Shock Lesion Only in Cardiogenic Shock (CULPRIT-SHOCK) 706 patients with multivessel disease, acute MI and cardiogenic shock. Randomized to: PCI of culprit vessel only or multivessel PCI. Primary End Point: Death or severe renal failure within 30 days. Thiele et al., NEJM (2017) 6
12/1/17 ORBITA: Design Chronic stable angina patients with 70% or more stenosis in a single vessel. 6 week period of medical optimization. Randomized 200 patients: 105 PCI/OMM, 95 OMM alone. OMM alone group (no PCI) got a sham PCI. Primary End Point: Change in exercise time at 6 weeks. Secondary End Point: Change in DSE wall motion score. Al-Lamee et al., Lancet, Published online November 2, 2017. ORBITA: Results Immediate Press Coverage of ORBITA PCI Placebo Exercise Time Increment 28.4 s 11.8 s p=0.2 Duke Treadmill score Inc. 1.22 0.10 p=0.1 Wall motion score (DSE) improved significantly in PCI group v. Placebo, p=0.0011. 7
12/1/17 Considerations Regarding ORIBITA Lancet “Comment” Accompanying ORBITA Article Strengths: Double-blind; Randomized; Sham operated control. Limitations: Small sample size with trend toward benefit (beta error?); short term outcome (6 weeks); 29% of patients had FFR greater than 0.80 and 32% had iFR greater than 0.89; cross over (four patients in placebo group got stents); 62% of patients had relatively mild angina (CCS class I or II); study only included chronic stable angina patients with single vessel disease. Positive Finding: Stents significantly reduced ischemia (improved wall motion score on stress echo: p value 0.0011). Brown and Redberg, Lancet, Published on Line November 2, 2017. www.thelancet.com FAME-2 Clinical Outcomes at Three Years in FAME 2 Trial 888 patients with chronic stable angina. 888 patients with chronic stable angina and at least one vessel with FFR of 0.80 or less. Randomized to best medical therapy or FFR guided stent treatment plus medical therapy. Randomized to: Stents plus MT for all lesions with FFR of 0.80 or less (447), or MT alone (441). Nearly 60% had single vessel disease. Registry: patients with FFR >0.80 for all lesions. Primary end point – Death, non-fatal MI or unplanned hospitalization leading to urgent revascularization (MACE). Primary end point: MACE (death, MI, urgent revascularization). 8
12/1/17 MACE in Patients with Chronic Stable Angina: FFR Angina Control in Patients with Chronic Stable Guided PCI v. Medical Therapy Alone Angina: FFR Guided PCI v. Medical Therapy Alone Fearon et al., (2017) Circulation (published online). Fearon et al., (2017) Circulation (published online). Cost Comparison: FFR Guided PCI v. Medical Conclusions Therapy Alone • IRA PCI alone, for almost all patients with STEMI and MVD. • Avoid acute MV PCI in STEMI patients with MVD. • Staged PCI of non-infarct lesions is preferable. • Above points are also true for patients in cardiogenic shock. • There is still a role for PCI in patients with chronic stable angina. Fearon et al., (2017) Circulation (pub. online). 9
Recommend
More recommend