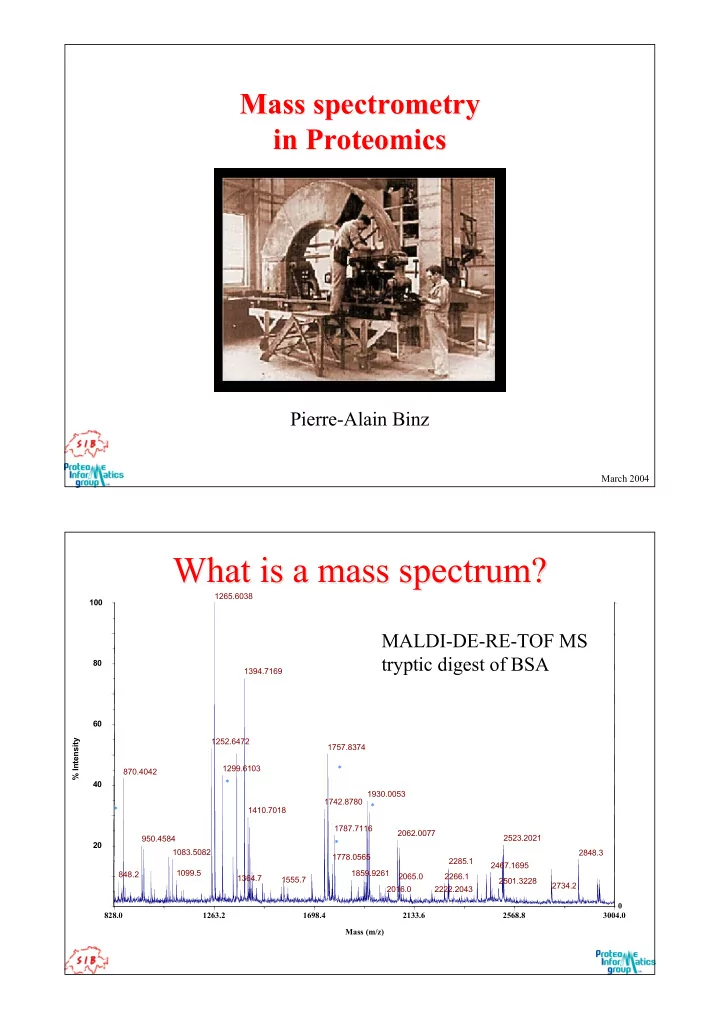

Mass spectrometry Mass spectrometry in Proteomics Proteomics in Pierre-Alain Binz March 2004 What is a mass spectrum? What is a mass spectrum? 1265.6038 100 MALDI-DE-RE-TOF MS tryptic digest of BSA 80 1394.7169 60 1252.6472 % Intensity 1757.8374 1299.6103 * 870.4042 * 40 1930.0053 1742.8780 * * 1410.7018 1787.7116 2062.0077 2523.2021 950.4584 * 20 1083.5082 2848.3 1778.0565 2285.1 2467.1695 1099.5 1859.9261 848.2 2065.0 2266.1 1364.7 1555.7 2501.3228 2734.2 2016.0 2222.2043 0 828.0 1263.2 1698.4 2133.6 2568.8 3004.0 Mass (m/z)
Protein Identification using Mass Spectrometry protein from gel/ tryptic digestion & 1-DE, 2-DE, PVDF/LC fraction peptide extraction LC T Y GGAAR EHI C LLGK PSTTGVE M FR G ANK unmodified and modified peptides Mass spectrometry, peptide mass fingerprints PMF identification MS Fragmentation Mass spectrometry, MS/MS identification peptide MS fragments
How are mass spectra produced ? ? How are mass spectra produced • Ions are produced in the source and are transferred into the mass analyser • They are separated according to their mass/charge ratio in the mass analyser (e.g. Quadrupole, Ion Trap, Time of Flight) • Ions of the various m/z values exit the analyser and are ‘counted’ by the detector Generic description of a mass Generic description of a mass spectrometer spectrometer Vacuum System Vacuum System Atmosphere Sample Ionisation Mass Data Sample Ionisation Mass Data Detector Detector Inlet Method Analyser System Inlet Method Analyser System
Ionization methods methods Ionization Analytes are ionized to be driven in the mass analyzer Electron impact (EI) Chemical Ionisation (CI) Fast atom bombardment (FAB) Field desorption (FD) Atmospheric Pressure Chemical Ionisation (APCI) ESI Electro-Spray Ionization MALDI Matrix Assisted Laser Desorption Ionization EI electron impact ionisation: beam of electrons through the gas-phase sample. Produces molecular ions or fragment ions. Typically 70eV. Sample heated. + Reproducible, structural information - sample must be volatile and stable, molecular ion often abscent mass range: < 1000Da CI: chemical ionisation: reagent gaz (methane, isobutane, or ammonia) ionized with electrons. High gaz pressure: (R = reagent, S = sample, e = electron, . = radical electron , H = hydrogen) R + e ---> R+. + 2e R+. + RH ---> RH+ + R. RH+ + S ---> SH+ + R Heated sample. + [M+H]+ often visible, less fragmentation than EI - sample must be volatile and stable, less structural info than EI mass range: < 1000Da DCI: Desorption CI : CI on a heated filament + rapid, simple - reproducibility mass range <1500Da NCI: negative-ion CI: electron capture; use of Methane to slow down electrons + efficient, sensitive; less fragmentation that EI, CI - not all molecule compatible, reproducibility mass range <1000Da
FD: Field Desorption: sample deposited on filament gradually heated by electric field. Sample ionise by electron tunneling. Ions are M+ and [M+Na]+ + simple spectra, almost no background - sensitive to alkali, slow, volatile to desorb mass range <2000-3000Da FI: Field ionisation: sample introduced in gas phase (heaten or not), ionised by electron tunneling near the emitter. + simple spectra, almost no background - sample must be volatile mass range <1000Da FAB: fast atom bombardment: analyte in a liquid matrix (glycerol, etc.). Bombardment with fast atom beam (xenon at 6keV). Desorbtion of molecular ions, fragments and matrix clusters sample introduced liquid, or LC/MS + rapid, simple, good for variety of compounds, strong currents, high resolution - background, sample must be soluble in matrix mass range ~300-6000Da SIMS: soft ionisation: similar to FAB but with ion beam as gas (Ce+), allowing higher acceleration (energy) + idem FAB - idem FAB, target can get hotter, more maintenance mass range 300-13000Da ESI: electrospray ionisation: The sample solution is sprayed across a high potential difference (a few kilovolts) from a needle into an orifice in the interface. Heat and gas flows are used to desolvate the ions existing in the sample solution. ESI often produces multiply charged ions with the number of charges tending to increase as the molecular weight increases. High to low flow rates 1 ml/min to nl/min. + good for charged, polar or basic compounds, m/z ok for most MS, best for multiply charged ions, low background, controlled fragmentation, MS/MS compatible - complementary to APCI: not good for uncharged, non-basic, low-polarity compounds, low ion currents mass range <200’000Da APCI: atmospheric pressure CI: as in ESI, sample introduced in a high potential difference field. Uses a corona discharge for better ionisation of less polar molecules than in ESI. APCI and ESI are complementary MALDI: Matrix-Assisted Laser Desorption Ionization: analyte co-crystallised in matrix. The matrix chromophore absorbs and distribute the energy of a laser, produced a plasma, vaporates and ionize the sample. + rapid, convenient for molecular weight (singly charged ions mostly) - MS/MS difficult, almost not compatible with LC coupling <500’000Da
Electrospray Ionization (ESI) Electrospray Ionization (ESI) pump S S SH + S S + + + + + MH + S + + MH + + MH S SH + + + + ++ + + S 2+ + 2+ + S S + + S n H MH 2 MH 2 S Smaller Coulomb explosion: Ions droplet droplet Clusters and ionic species Modif. From Alex Scherl Matrix Assisted Laser Desorption Desorption/Ionization /Ionization Matrix Assisted Laser MALDI MALDI UV or IR laser sample target Membrane, gel or metal Matrix grid Analytes
Matrix Assisted Laser Desorption Desorption/Ionization /Ionization Matrix Assisted Laser MALDI MALDI Mass Analyzers Analyzers Mass Mass Spectrometers separate ions according to their mass-to- charge (m/z) ratios – Magnetic Sector – Quadrupole – Ion Trap – Time-of-flight – Hybrid- Sector/trap, Quad/TOF, etc.
Quadrupole mass analyzer Quadrupole mass analyzer + + + + RF + DC The quadrupole consists of • The ion is transmitted along the two pairs of parallel rods with quadrupole in a stable trajectory Rf field. applied DC and RF voltages. The ion does not have a stable Ions are scanned by varying trajectory and is ejected from the the DC/Rf quadrupole quadrupole. voltages. Ion Trap mass analyzer Ion Trap mass analyzer • Consists of ring electrode and two end caps • Principle very similar to quadrupole • Ions stored by RF & DC fields • Scanning field can eject ions of specific m/z • Advantages • - MS/MS/MS….. • - High sensitivity full scan MS/MS
Time of Flight (TOF) mass analyzer Time of Flight (TOF) mass analyzer High vacuum flight tube Ion source Detector time 1 Small ions are faster than heavy, and reach detector time 2 first time 3 Ion source High vacuum flight tube Detector Reflectron
FTMS Ions moving at their cyclotron frequency can absorb RF energy at this same frequency. A pulse of RF excites the ions in the magnetic field. The ions re-emit the radiation, which is picked up by the reciever plates. The decay produces a free-induction decay signal that can be Fourier transformed to produce the emitted frequencies, and therefore the masses of the ions present. FTMS FTMS
What is a mass spectrum? What is a mass spectrum? 1265.6038 100 MALDI-DE-RE-TOF MS tryptic digest of BSA 80 1394.7169 60 1252.6472 % Intensity 1757.8374 1299.6103 * 870.4042 * 40 1930.0053 1742.8780 * * 1410.7018 1787.7116 2062.0077 2523.2021 950.4584 * 20 1083.5082 2848.3 1778.0565 2285.1 2467.1695 1099.5 1859.9261 848.2 2065.0 2266.1 1364.7 1555.7 2501.3228 2734.2 2016.0 2222.2043 0 828.0 1263.2 1698.4 2133.6 2568.8 3004.0 Mass (m/z) How does a peptide signal looks like? How does a peptide signal looks like? Low resolution High resolution
Isotopic distribution Isotopic distribution Mass resolution 0.1% vs. 1 ppm Symbol Mass Abund. Symbol Mass Abund ------ ---------- ------ ------ ----------- ------- C(12) 12.000000 98.90 C(13) 13.003355 1.10 N(14) 14.003074 99.63 N(15) 15.000109 0.37 O(16) 15.994915 99.76 O(17) 16.999131 0.038 H(1) 1.007825 99.99 H(2) 2.014102 0.015 S(32) 31.972072 95.02 S(33) 32.971459 0.75 Isotopic distribution Isotopic distribution
Mass resolution 10002000Half massFull width Mass resolution 0.5 FWHM 1.0 FWHM 0.7 FWHM 0.3 FWHM 0.2 FWHM 0.1 FWHM
524.3 100 95 90 85 80 Singly charged Ion: 75 Singly charged Ion: 70 Distance between Peak Distance between Peak 65 and Isotop Isotop 1 1 amu amu and Relative Abundance 60 55 50 45 _ = 1.0 amu _ = 1.0 amu 40 35 30 525.3 _ = 1.0 amu _ 25 = 1.0 amu 20 15 10 526.2 5 0 520 521 522 523 524 525 526 527 528 529 m/z 262.6 100 95 90 85 80 75 Doubly charged Ion: Doubly charged Ion: 70 Distance between Peak and Distance between Peak and 65 Isotop 0.5 Isotop 0.5 amu amu Relative Abundance 60 55 50 45 _ = 0.5 amu _ = 0.5 amu 40 35 30 25 263.1 20 _ = 0.5 amu _ = 0.5 amu 15 10 263.6 5 0 258 259 260 261 262 263 264 265 266 267 m/z
Recommend
More recommend