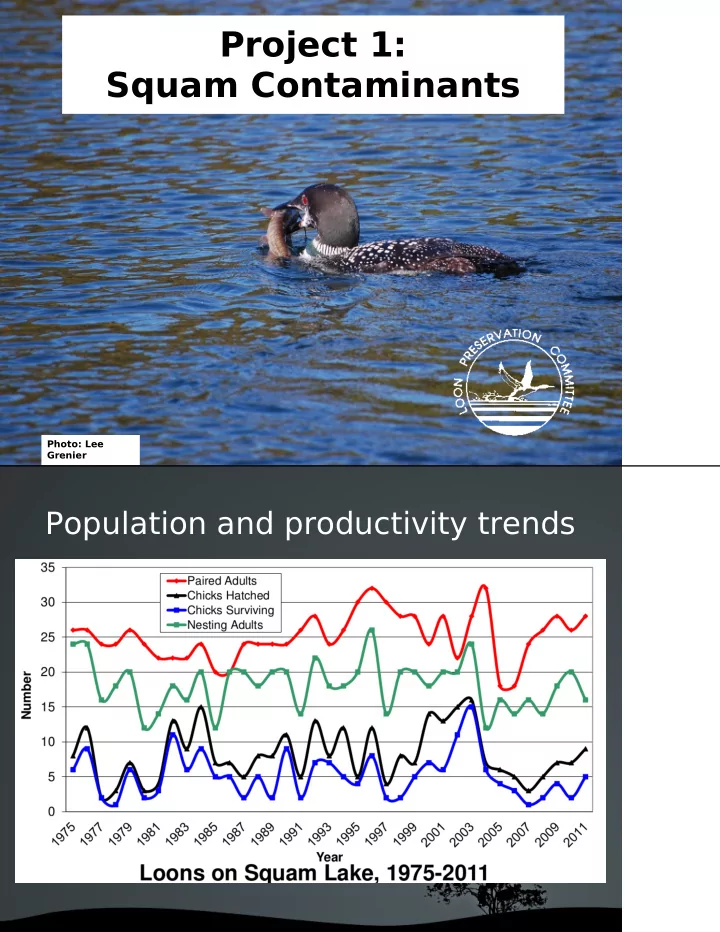

Project 1: Squam Contaminants Photo: Lee Grenier Population and productivity trends
S q u a m L a k e L oon s O v e r T im e 16.0 Avg 1995-2004 14.0 Avg 2005-2007 12.0 Avg 2008-2012 10.0 8.0 6.0 4.0 2.0 0.0 Territorial Pairs Nesting Pairs C hicks Hatched C hicks S urviving Organic Pollutants
Inorganic Pollutants Student Data
Project Possibilities: Field Based • Sediment sampling • Water sampling • Possible Sources – Squam – Reference Lakes – **Streams flowing into Squam **Useful focus for the LPC Project Possibilities: Instrument Based • **Analysis of Zinc by flame AA and IC **Useful focus for the LPC
Project 2: Project 2: Affects of Aquatic Plants on Affects of Aquatic Plants on Water / Sediment Chemistry Water / Sediment Chemistry Figures from: http://www.missouriplants.com/Others/Myriophyllum_heterophyllum_plant.jpg https://c53a19f696-custmedia.vresp.com/library/1306354496/e2b7d3b0d8/uconn_ipane_myriospica_03c-reduced.JPG Native & Non-Native Native & Non-Native Aquatic Plants Aquatic Plants Natives Non-Natives Bladderwort Brazillian elodea Coontail Euarasion Water-Milfoil Fanwort Variable Water-Milfoil Native Water Milfoil Pondweed Waterweed
Lab-based Project Lab-based Project ● Plants grown in tanks Plants grown in tanks ● Control and Experimental tanks Control and Experimental tanks ● Monitor water quality Monitor water quality ● Analyze water, sediment, plants Analyze water, sediment, plants Class Research Project Analyses Analytes Water Quality Anions: Chloride, Fluoride, Alkalinity Nitrate, Phosphate, etc Conductivity Cations: Calcium, Sodium, Dissolved Oxygen Potassium, Magnesium, etc. Hardness Metals: Lead, Cadmium, pH, pE Strontium, Aluminum, Zinc Total Dissolved Solids Techniques Ion Chromatography Ion Selective Electrodes UV-Vis Spectrophotometry Titration Atomic Absorption 12 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University
Project Timeline ● Mar 24: Time in lab ● April 6: Project Proposal due ● April 14-May 5: Time in lab ● Finals Week: Project Presentation 13 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University Quality Assurance of Results 14 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University
Quality Assurance ● Quality Assurance: Set of operating principles that, if strictly followed during sample collection and analysis, will produce data of known and defensible quality ● How do you do it? ● Eliminate contaminants and interferences during sample collection, prep, and analysis ● Prepare samples and standards to highest precision possible ● Use best equipment possible from start to finish ● Calibrate instruments to highest precision possible ● Run Quality Control checks during analysis ● Take care with data—significant figures, statistical analysis Make sure your answers are correct! 15 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University Quality Assurance Eliminating contaminants and interferences ● Use clean equipment ● Water should not “bead” on glassware ● If you do not know where it has been, it is dirty ● Final rinse of everything should be with Ultra-pure water ● Use purest reagents possible ● Check purity on bottle. ● Use Ultra-pure water for sample/standard prep 16 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University
Quality Assurance Highest Precision Samples/Standards ● Use best equipment Yes! No! Measuring Volume Volumetric flasks Beakers Volumetric pipettes Graduated cylinder Auto-pipettes Burets Measuring Mass 4-digit Analytical balance Anything less than 4 digits RECORD ALL DIGITS Rounding last digit or 2 17 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University Quality Assurance Quality Control Checks ● Blanks: prepare/analyze ultra-pure water same as samples. ● Should yield instrument response essentially equal to zero. If not, subtract this value from sample values ● Duplicates: Run all samples and standards at least twice. ● Should vary by no more than 5% ● Difference in duplicates governs precision of answer (sig figs) ● Average of values used in final analysis ● Internal Standards: Create/Analyze knowns same as sample ● Separate from standards ● Run periodically throughout a long set of analyses ● Spike recovery: Add a known amount of analyte to a sample 18 CH 3600 / ESP 5090: Environmental Chemistry, Plymouth State University
Recommend
More recommend