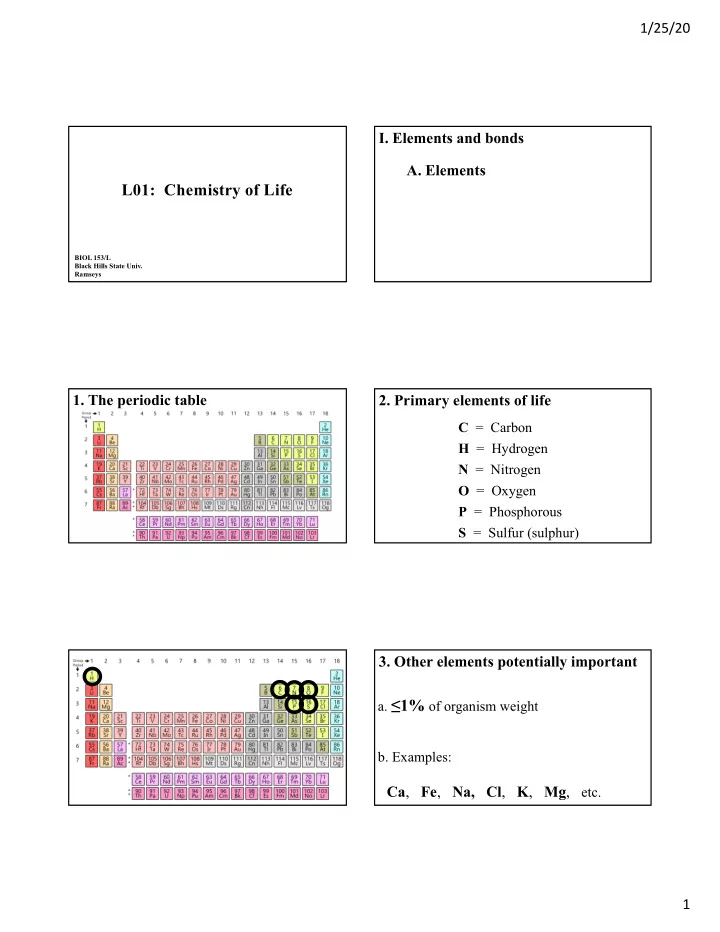

1/25/20 I. Elements and bonds A. Elements L01: Chemistry of Life BIOL 153/L Black Hills State Univ. Ramseys 1. The periodic table 2. Primary elements of life C = Carbon H = Hydrogen N = Nitrogen O = Oxygen P = Phosphorous S = Sulfur (sulphur) 3. Other elements potentially important a. ≤ 1% of organism weight b. Examples: Ca , Fe , Na, Cl , K , Mg , etc. 1
1/25/20 I. Elements and bonds B. Bonds 1. Elements bond to make molecules 2. Each element can make a set number of bonds (connections) O a. Carbon has 4 bonds C O b. Hydrogen has 1 bond H H O c. Oxygen has 2 bonds Water (H 2 O) Carbon dioxide (CO 2 ) 2. Number of bonds I. Elements and bonds O C. Organic chemistry 2 bonds 1 bond 1 bond C O 2 bonds H H O Water (H 2 O) Carbon dioxide (CO 2 ) 2
1/25/20 1. Organic chemistry focuses on CHNOPS 2. Organic molecules built on carbon C = Carbon a. Number of C's vary H = Hydrogen N = Nitrogen O = Oxygen b. "Chains" or "rings" P = Phosphorous S = Sulfur 3. Elements attached to carbon vary 4. Main types of organic molecules a. Carbohydrates a. Hydrogen... commonly attached (-H) b. Lipids b. Oxygen... often attached (=O or -OH) c. Other elements... can be attached c. Proteins d. Together... make organic molecule d. Nucleic acids II. Carbohydrates A. Significance of carbohydrates 1. Most abundant organic molecules "Carbon with water added" 2. Main energy source (transport + storage) 3. Structural functions 3
1/25/20 B. Monosaccharides = simple sugars 1. Elemental composition a. Ratio = 1 carbon : 2 hydrogen : 1 oxygen b. Number of C's = 3-7 2. Examples of monosaccharides Lots of H and O and OH! ( parts of water) 3-carbon 5-carbon 6-carbon sugar sugar sugar 3. Examples of glucose (chain vs. rings) 3. Examples of glucose (chain vs. rings) C C C C C C 4
1/25/20 3. Examples of glucose (chain vs. rings) 3. Examples of glucose (chain vs. rings) 3. Examples of glucose (chain vs. rings) 3. Examples of glucose (chain vs. rings) a. All glucose forms have 6 carbons b. Different ring "versions" c. Form influences chemical rxns 5. Building blocks for other carbohydrates 4. Monosaccharides association w/ water a. Hydrophilic = "water loving" Monos bond to make di- and poly-saccharides b. Lots of H's and OH's c. Dissolve in water 5
1/25/20 C. Disaccharides = two sugars 6. Association with photosynthesis/respiration a. Plants... make glucose (photosynthesis) b. Animals... use glucose (respiration) c. Vertebrates... transport glucose (in blood) 1. Disaccharide examples Sucrose • Table sugar a. Sucrose • Made by plants (1º transported sugar) • Glucose + fructose Sugar cane – a grass • Developed in India (800s) • Brought to New World (1400s) • Encouraged slave trade • Grown in tropical regions 6
1/25/20 Sugar beets – Beta , an amaranth • Developed in Germany (1700s) • Napoleon motivated development • Japanese internment => interior U.S. • Grown in temperate regions U.S. sugar tariffs: #454 Lollipop War Planet Money D. Polysaccharides = many sugars b. Lactose • Milk sugar • Glucose + galactose • Lactase breaks bond between sugars • 75% of world population is lactose intolerant 7
1/25/20 1. Polysaccharide structure 2. Polysaccharide examples a. Long monosaccharide chains a. Starch b. Cellulose b. Don't dissolve easily in water c. Chitin 3. Starch Amylose (a plant starch) a. Glucose chains (folded/compact) b. 1º storage carb in plants c. Somewhat difficult to break d. Amylose vs. amylopectin (plant starches) Amylopectin (a plant starch) Which is harder for humans to digest? 8
1/25/20 Which is harder for humans to digest? Examples of amylose-rich foods: • Long chain harder to digest than branched • Long-grain rice • Amylose is resistant starch • Starchy potatoes • Amylose-rich foods raise insulin less • Corn, oats, wheat, legumes; bananas (vs. amylopectin-rich foods) Examples of amylopectin-rich foods: Enzyme digestion: • Amylase • Short-grain rice • Waxy potatoes • Salivary glands and pancreas • White flour products 4. Cellulose e. Glycogen (an animal starch) a. Glucose chains b. Plant cell walls ("fiber" in wood, cotton) • Glucose storage in animals (liver) c. Most abundant carbon compound • 1 st energy released w/ exertion d. Few organisms can digest 9
1/25/20 4. Cellulose 5. Chitin a. Fungal cell walls b. Invertebrate exoskeletons c. Animal connective tissue (in part) 5. Chitin III. Lipids "fat and fat-like substances" A. Significance of lipids B. Triglycerides = glycerol + fatty acids 1. High-energy C-H bonds 1. Energy storage (triglygerides) 2. Most animal energy storage ("fat") 2. Structures (wax, phospholipids) 3. Some plant storage (seeds, fruits) 3. Hydrophopic (water-fearing) 4. Glucose convert-able to triglycerides 10
1/25/20 Carbon-hydrogen bonds— LOTS of energy! C. Saturated vs. unsaturated fats • Difference à fatty acids tails • Saturated à only single bonds (C-H) • Unsaturated à 1+ double bonds (C=C) Saturated fats • Solid: tails flexible, pack tightly • Resistant: C-H bonds stable • "Bad": increase cholesterol + triglycerides • Examples: coconut, palm; lard, tallow 11
1/25/20 Unsaturated fats Key polyunsaturated fats (Omega-3, -6) • Liquid: tails kinky, pack loosely • Modern diet: high O-6, low O-3 • Rancidity: C=C bonds unstable • O-3 sources: algae, fish, flax, walnuts, canola • "Good": varied health effects • O-6 sources: vegetable oils (soybean, corn) • Examples: fruit + vegetable oils D. Phospholipids D. Phospholipids 1. Phosphate group + lipid 2. Hydrophilic part (polar head) and hydrophobic part (non-polar tail) 3. Plasma membrane 12
1/25/20 Attracts water Repels water When surrounded by water... • Polar heads è outward (into water) • Nonpolar tails è inward (away from water) Plasma (cell) membrane E. Waxes, suberin, cutin 1. Cell wall components 2. Reduce water loss F. Steroids, sterols IV. Proteins 1. Stabilize phospholipid tails "action molecules of the cell" 2. Hormones 3. Only animals have cholesterol! 13
1/25/20 A. Significance of proteins B. Structure and origin 1. ≥ 50% of biomass of non-plant organisms 2. Building blocks of tissues; diverse functions (enzymes, hormones, etc.) 1. Amino acids lots of nitrogen "organics w/ amine (-NH 2 ), carboxyl (-COOH) and side chain (-R); connect to make proteins" 14
1/25/20 2. Folding "complex structure created by bonding among amino acids" V. Nucleic acids "hereditary blueprints for the cell" B. Structure A. Significance of nucleic acids 1. Compose DNA and RNA DNA + RNA can have complex structure; 2. Genetic code to make proteins discussed later in class! 3. Modified to make energy carriers (ATP + NADH/NADPH) 15
1/25/20 ATP P.S. Energy "currency" in the cell A. ATP B. NADH / NADPH NADH / NADPH 16
Recommend
More recommend