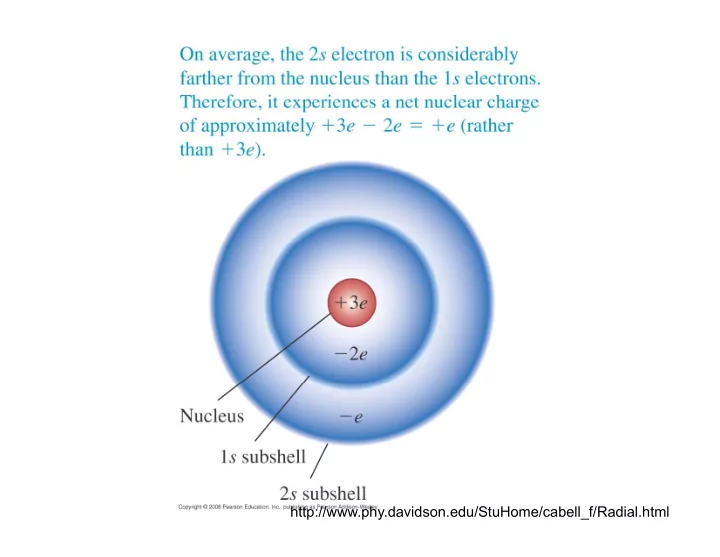

http://www.phy.davidson.edu/StuHome/cabell_f/Radial.html
r e/pm D /(kJ · mol − 1) Bond r e/pm D /(kJ · mol − 1) Bond H—H 74.130 432.00 C—H 112.0 335 H—D 74.140 435.39 N—H 103.8 310 D—D 74.143 439.53 P—H 143.3* 340 C—C 131.2 603 O—H 97.1 425 N—N N N 109 76 109.76 942 942 F F—H H 91 7 91.7 564 564 P—P 189.3 485 Cl—H 127.5 428 O—O 120.741 493.6 Br—H 140.8 362 S—S 188.7 423 I—H 160.0 295 F—F 141.8* 155 C—N 117.7 745 Cl—Cl 198.8 239 C—O 113.1 1069 Br—Br 228.4 190 N—O 115.02 627 I—I I I 266.7 266 7 147 147 Cl Cl—F F 162 8 162.8 247 247
Q42.1 The difference between an ionic bond and a covalent bond is The difference between an ionic bond and a covalent bond is A. ionic bonds are only found in crystals such as sodium chloride (NaCl) where there are many atoms in close ( ) y proximity. B. covalent bonds are only found in molecules with three or more atoms. C. ionic bonds are highly directional, while covalent b bonds are not. d D. ionic bonds involve the transfer of an electron from one atom to another one atom to another, while covalent bonds involve hile co alent bonds in ol e electrons that spend much of their time between atoms.
Q42.3 This diagram shows the vibrational and rotational energy levels of a diatomic molecule. Consider two possible transitions for this molecule: possible transitions for this molecule: A. n = 2, l = 5 to n = 1, l = 4 B n = 2 l = 1 to n = 1 l = 0 B. n = 2, l = 1 to n = 1, l = 0 The energy change is greater for A. transition A. B. transition B. C. The energy change is the same for both transitions. C. The energy change is the same for both transitions. D. any of the above, depending on circumstances.
Recommend
More recommend