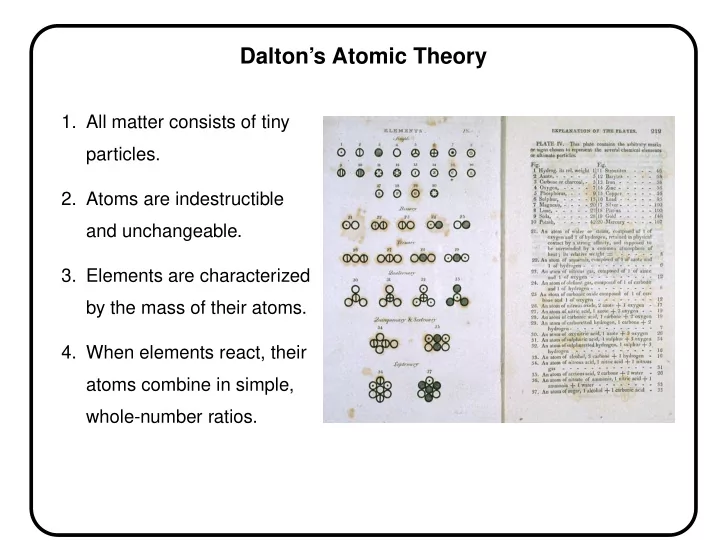

Dalton’s Atomic Theory 1. All matter consists of tiny particles. 2. Atoms are indestructible and unchangeable. 3. Elements are characterized by the mass of their atoms. 4. When elements react, their atoms combine in simple, whole-number ratios.
Trajectory of Brownian Motion
Specific Heats of Gases 35 C V (J/K-mole) SO 2 30 CO 2 H 2 O CH 4 25 Cl 2 20 H 2 N 2 O 2 CO 15 He Ar Ne Kr 10 5 0 Molecule
Specific Heats of Gases 35 C V (J/K-mole) SO 2 30 7 2 R CO 2 H 2 O CH 4 25 Cl 2 5 2 R 20 H 2 N 2 O 2 CO 15 3 2 R He Ar Ne Kr 10 5 0 Molecule
Specific Heats of Gases
Extrapolating to Absolute Zero 8 6 4 Trials 2 0 � 300 � 250 � 200 � 150 � 100 � 50 0 Absolute Zero � centigrade �
Fitting the Data 70 60 Variance 50 40 30 Estimated 20 10 0 0.27 0.28 0.29 0.3 0.31 0.32 Slope In the plot above the value of the y-intercept is kept at its best fit value and the slope is varied. The estimated variance is the following. � N i =1 ( y i − ( mx i + b )) 2 σ 2 = (1) N − d.o.f where N is the number of data points and d.o.f is the number of degrees of freedom ( i.e. free parameters) in the fit.
Recommend
More recommend