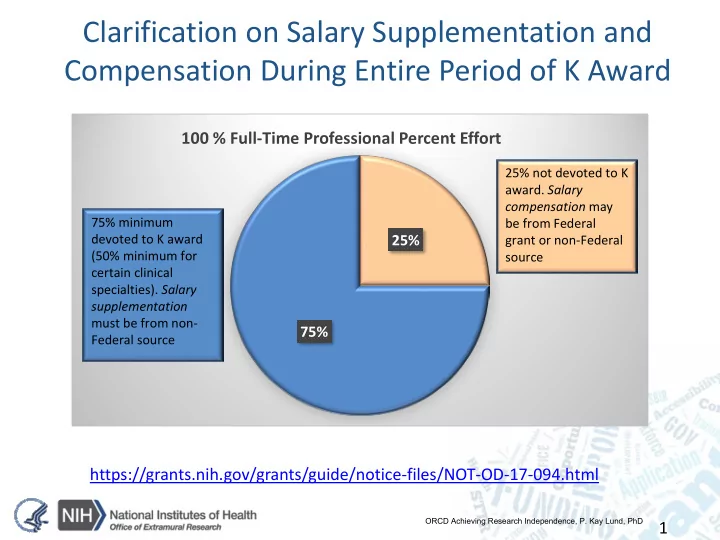

Clarification on Salary Supplementation and Compensation During Entire Period of K Award 100 % Full-Time Professional Percent Effort 25% not devoted to K award. Salary compensation may 75% minimum be from Federal devoted to K award 25% grant or non-Federal (50% minimum for source certain clinical specialties). Salary supplementation must be from non- 75% Federal source https://grants.nih.gov/grants/guide/notice-files/NOT-OD-17-094.html ORCD Achieving Research Independence, P. Kay Lund, PhD 1
Reduced Effort, Salary Supplementation and Compensation During Last 2 yrs of K Award 100 % Full-Time Professional Percent Effort 25% not devoted to K award. Salary compensation may be from Federal grant or 25% 50% minimum non-Federal source devoted to K award. Salary 50% supplementation 25% research and must be from non- Salary compensation Federal source 25% only if PD/PI on Federal grant ORCD Achieving Research Independence, P. Kay Lund, PhD 2
Rigor & Reproducibility Updates 3 ORCD Achieving Research Independence, P. Kay Lund, PhD
NIH Weekly Table of Contents https://grants.nih.gov/Grants/guide/listserv.htm NIH Guide LISTSERV: Subscribe to Weekly TOC E-Mail with New NIH Guide Postings • Useful way to receive weekly emails (usually on Friday afternoon) with the Current Weekly Table of Contents (TOC) from the NIH Guide to Grants and Contracts • Direct links to all funding opportunities and notices published during each week • Information on new programs & RFAs • Many early stage investigators do not access 4 ORCD Achieving Research Independence, P. Kay Lund, PhD
Congress & the NIH Next Generation Researcher Initiative (NGRI) “The Director of the National Institutes of Health shall … develop, modify, or prioritize policies, as needed … to promote opportunities for new researchers and earlier research independence , such as policies to increase opportunities for new researchers to receive funding , enhance training and mentorship programs for researchers, and enhance workforce diversity .” ORCD Achieving Research Independence, P. Kay Lund, PhD 5
Updates on NIH Next Generation Researchers Initiative (NGRI) • Presented at June 2017 NIH Advisory Committee to the NIH Director (ACD) • NIH Guide notice (NOT-OD-17-101) published August 31, 2017: Policy Supporting the Next Generation Researchers Initiative • Enhance support for Early Stage Investigators • Being refined with feedback from NIH leadership, and ongoing input from an NGRI ACD working group • Input from an NIH supported National Academies of Sciences, Engineering and Medicine (NASEM) NGRI study anticipated in April 2018. ORCD Achieving Research Independence, P. Kay Lund, PhD 6
Early Stage Investigators • Early stage investigator or ESI is a Program Director/Principal Investigator (PD/PI) who has completed their terminal research degree or end of post-graduate clinical training **, whichever date is later, within the past 10 years and who has not previously competed successfully as PD/PI for a substantial NIH independent research award. • Multiple PI grants : ALL must be ESI to qualify for ESI status Ensure ESI status is up to date ** Clinicians should update their eRA commons profile if it currently has the end date of medical residency and provide the end date of postgraduate clinical training including fellowship.** ORCD Achieving Research Independence, P. Kay Lund, PhD 7
Questions re gender & funding • Funding rates for women (22.3%) and men (23.9%) similar in 2016 • Between 2009 and 2016 R01 applications from women increased by 96.4% while those from men increased by 49.7% • In the same period R01 awards to women increased by 69.4% while those to men increased by 25.8% • Age to first R01 and renewal is similar for men and women • R01 applicants between 2009-16: women represent 37.9% of ESI applicants, 32.1% of New Investigators (NI) & 27.2% of established investigators • R01 awardees: women 36.2% ESI, 33.9% NI and 26.5% established investigators ORCD Achieving Research Independence, P. Kay Lund, PhD 8
Strategic Career Planning • Plan strategically when approaching the end of funding and need for new application or renewal – Conserve resources – Discuss, identify, request sources of bridging funds • Ensure protected time or assistance to achieve goals (if you don’t ask you will not receive) – Time to write/complete publications and grants – Obtain preliminary data (time, resources, staff) – Ask for relief from non-essential activities/committees • Consult with program officers (and others) for guidance on unfunded grants and response to summary statement in a revision • Apply to foundations for funding ORCD Achieving Research Independence, P. Kay Lund, PhD 9
New Alternate Funding Sources OnPar provides innovative and exceptional research applications with a second opportunity for funding. – Applicants : submit their highly scored unfunded applications to OnPAR – Members : review research projects and select those that match their research priority areas for potential partnering/funding. – Partners: manage the review https://onpar.leidosweb.com process of research applications that are submitted to OnPAR. ORCD Achieving Research Independence, P. Kay Lund, PhD 10
Remember and Emphasize Biomedical Research Career Positives Science Long-term, constant exciting discoveries (own and the field) Flexible and rewarding positions in academics and other Academics research careers Mentees A family of former trainees, mentees across the USA and beyond Yes! Amazing rewards of their achievements Writing a grant is itself a reward (& fun) Funding Immersion and expertise in the topic • Focus on more than the $$ • 11 ORCD Achieving Research Independence, P. Kay Lund, PhD
ORCD Achieving Research Independence, P. Kay Lund, PhD 1 2
New Clinical Trials Policy NIH Definition of a Clinical Trial A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Applications/proposals involving clinical trials with due dates on or after January 25, • 2018 must be submitted to a funding opportunity announcement (FOA) or request for proposal (RFP) that explicitly states it will accept clinical trials. • All existing FOAs will be updated with the following changes: • All clinical trial FOAs will specify allowability of clinical trials in the FOA title (required, not allowed, optional) FOAs will specify the allowability of clinical trials in Section II. Award Information • FOAs that accept clinical trials will incorporate specific review criteria to ensure that • reviewers appropriately consider clinical trial-related information • https://grants.nih.gov/policy/clinical-trials/definition.htm K awards permit independent clinical trials – apply to the correct FOA • T/F awards permit mentored ‘clinical trials research experience’ but not independent • clinical trials led by trainee or fellow ORCD Achieving Research Independence, P. Kay Lund, PhD 13
Request an Extension of ESI Status § PD/PIs may experience a lapse in their research or research training or have circumstances that necessitate less than full-time effort during their ESI status. § The NIH will consider requests to extend ESI status for medical concerns, disability, family care responsibilities, extended periods of clinical training, natural disasters, and active duty military service. § Extensions are determined on a case by case basis at the discretion of NIH. § ESIs may request an extension of their eligibility under existing ESI procedures (NOT-OD-09-034) described in the Extension Request Form. ORCD Achieving Research Independence, P. Kay Lund, PhD 1 4
Compare Funding & Success Rates • Success rates The percentage of reviewed grant applications which receive funding. • Computed on a fiscal year basis and include applications that are peer reviewed and either scored or • unscored by an Initial Review Group. Determined by dividing the number of competing applications funded by the sum of the total number of • competing applications reviewed. Funded grants, which had one or more submissions for the same project in the same fiscal year, are only • counted once. Funding rates • • Person-based rather than application-based statistic • Higher than success rate • Counts applicants as funded whether they receive one or multiple awards in the same fiscal year. • The numerator is the number of applicants receiving any funding that fiscal year The denominator is the number of applicants for that fiscal year. • Funding rates, which are higher than success rates, paint a more promising picture for your prospects of • receiving NIH funding. ORCD Achieving Research Independence, P. Kay Lund, PhD 1 5
Grants and Funding http://grants.nih.gov/grants/oer.htm Find Funding: NIH Guide for Grants and Contracts § How to Apply: Due Dates, Submission Policies, Prepare to Apply § and Register, Format and Write, Submission Process Explore NIH Funded Research - Research Portfolio Online § Reporting Tools (RePORT) ORCD Achieving Research Independence, P. Kay Lund, PhD 16
Recommend
More recommend