A clinical decision support tool to aid in the management of - PowerPoint PPT Presentation
A clinical decision support tool to aid in the management of hospitalized cirrhotic patients Osman Ahmed MD & Mayur MD FRCPC Department of Medicine: Division of Gastroenterology Toronto General Hospital Toronto, Canada Background The
A clinical decision support tool to aid in the management of hospitalized cirrhotic patients Osman Ahmed MD & Mayur MD FRCPC Department of Medicine: Division of Gastroenterology Toronto General Hospital Toronto, Canada
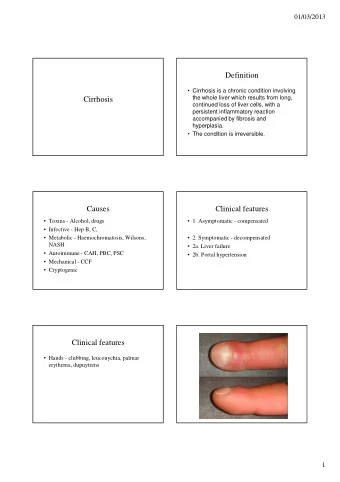
Background • The complications from cirrhosis often require hospitalizations that are prolonged and expensive (1.4 billion per year in USA). • No change in inpatient mortality between 2002-2011 (8.6% and 9.1% respectively). • Adherence to diagnostic and therapeutic guidelines is low.
Background • Clinical Decision Support previously implemented in management of variceal bleed. ○ Increased overall optimal care from 41% to 65% ○ Increased prophylactic antibiotic use from 57% to 75% ○ Increased somatastatin analog use from 54% to 76% ○ Decreased 30-day readmission rate from 41% to 13% Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012. Bajaj JS, Reddy KR, Tandon P, Wong F, et al. The Three-Month Readmission Rate Remains Unacceptably High in a Large North American Cohort of Cirrhotic Patients. Hepatology. 2015. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013. Johnson EA, Spier BJ, Leff JA, Lucey MR, Said A. Optimising the care of patients with cirrhosis and gastrointestinal haemorrhage: a quality improvement study. AlPT. 2013.
Innovation • First comprehensive standardized order set with associated decision support to guide initial management of hospitalized patients with decompensated cirrhosis.
Aims • Primary Aim / Hypothesis: The utilization of a Liver Care Bundle with standardized order sets and decision support at time of admission will improve clinician adherence to guideline driven management of patients with complications of cirrhosis. • Secondary Aim: To assess if the Liver Care Bundle decreases length of stay, decreases 30- day mortality, or decreases 30-day readmission rates in patients admitted with decompensated liver cirrhosis.
Methods: Study Design • Randomized Control Trial with intention to treat analysis. • Internal Medicine and Gastroenterology & Hepatology house staff will be enrolled and randomized into two teams working in parrallel taking care of two separate services: Control Team: Will not have access to Liver Care Bundle tool Experimental Team: Will receive access and education regarding how to utilize the standard order sets and access the decision support tool Patients will be randomized to either team
Liver Care Bundle Design ORDER ORDERS: S: [ x] Li ve r Ca r e Bundl e [ ] As c i t e s [ x] Ga s t r oi nt e s t i na l bl e e di ng [ x] Nur s e : Pl a c e 2 La r ge - bor e pe r i phe r a l I Vs ( 18 ga uge or l a r ge r ) [ x] La b: Type & Sc r e e n [ x] La b: CBC q8hr [ x] La b: I NR q8hr [ ] Bl ood pr oduc t s : pa c ke d RBC * [ ] Bl ood pr oduc t s : pl a t e l e t s * [ ] Bl ood pr oduc t s : f r e s h f r oz e n pl a s m a * [ x] Di e t : NPO [ x] M e d: pa nt opr a z ol e 40 m g I V BI D * [ x] M e d: oc t r e ot i de gt t ( 50 m c g l oa d, t he n 50 m c g/ hr ) f or 5 da ys * [ x] M e d: c e f t r i a xone 1gm I V da i l y f or 7 da ys * The use of short-term prophylactic [ ] Pa r a c e nt e s i s , s m a l l vol um e ( 50 m L) * antibiotics in patients with cirrhosis and GI [ ] La b: Ce l l Count hemorrhage with or without ascites has [ ] La b: Cul t ur e been shown not only to decrease the rate of [ ] La b: Gr a m s t a i n bacterial infections but also to increase [ ] La b: Al bum i n survival. Link to guideline. [ ] Pr oc e dur e : Es opha goga s t r oduode nos c opy * [ ] He pa t i c Enc e pha l opa t hy [ ] Spont a ne ous Ba c t e r i a l Pe r i t oni t i s [ ] Ac ut e Ki dne y I nj ur y
Budget Resource Purpose Cost (USD) Programming Development of code for $15,000 liver bundle Support Staff Research aid, support of $5,000 physicians Research Staff Salary support of research $60,000 fellow Computer equipment Development tools and $5,0000 software Statistician Statistical analysis $5,0000 Overhead Miscellaneous $20,000 Total Request Funding source: TBD $110,000
Reflections • Funding sources easiest part of starting project . • Institutional buy in was key. • Engaging stakeholders was difficult and time consuming.
Recommend
More recommend
Explore More Topics
Stay informed with curated content and fresh updates.