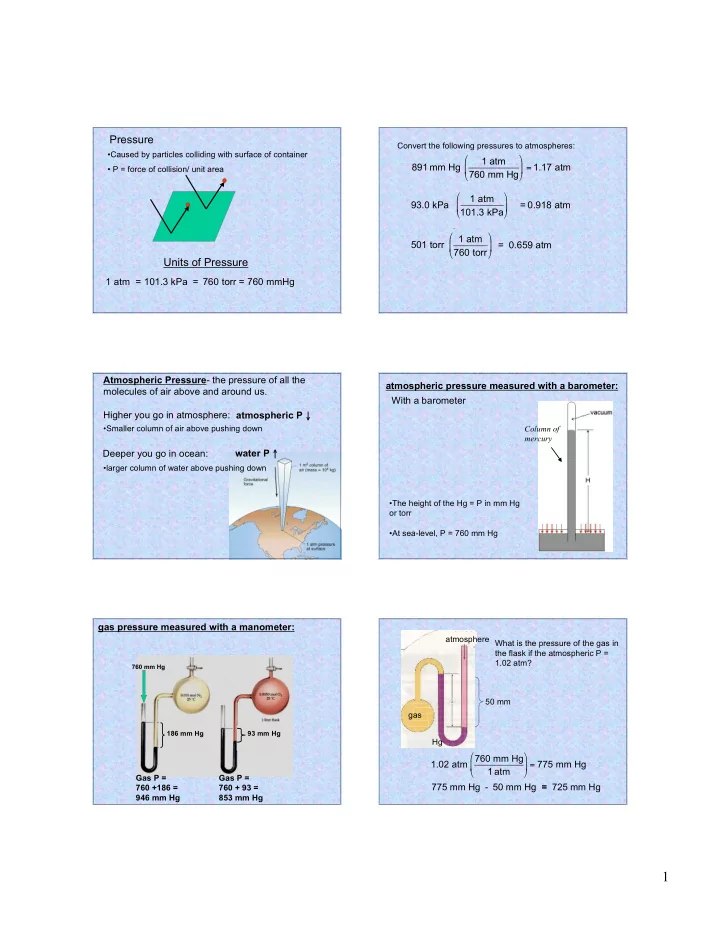

Pressure Convert the following pressures to atmospheres: •Caused by particles colliding with surface of container � � � 1 atm 891 mm Hg mm Hg � = 1.17 atm • P = force of collision/ unit area � � 760 mm Hg � � � � � 1 atm 93.0 kPa 93.0 kPa � = 0.918 atm � 101.3 kPa � � � � 1 atm � 501 torr 501 torr = 0.659 atm � � 760 torr � � Units of Pressure 1 atm = 101.3 kPa = 760 torr = 760 mmHg Atmospheric Pressure - the pressure of all the atmospheric pressure measured with a barometer: molecules of air above and around us. With a barometer Higher you go in atmosphere: atmospheric P ↓ •Smaller column of air above pushing down Column of mercury Deeper you go in ocean: water P ↑ •larger column of water above pushing down •The height of the Hg = P in mm Hg or torr •At sea-level, P = 760 mm Hg gas pressure measured with a manometer: atmosphere What is the pressure of the gas in the flask if the atmospheric P = 1.02 atm? 760 mm Hg 50 mm gas 186 mm Hg 93 mm Hg Hg � � � atm 760 mm Hg 1.02 atm � = 775 mm Hg � � 1 atm � � � Gas P = Gas P = 775 mm Hg - 50 mm Hg = 775 mm Hg 50 mm Hg = 725 mm Hg 760 +186 = 760 + 93 = 946 mm Hg 853 mm Hg 1
Gas Laws Gas temperatures must be in Kelvins for Boyle Boyle’ ’s Law s Law all calculations: PV = constant If T held constant Kelvins = ° C + 273 Kelvins = How are P and V of a gas related? Inversely related P 1 V 1 = P 2 V 2 Initial conditions = final conditions Charles’ ’s Law s Law Charles V • Only holds true if T in K T = constant and P held constant How are T and V of a gas related? Directly related V = V 1 2 T T 1 2 Combined Gas Law Combined Gas Law Avogadro’ Avogadro ’s Law s Law initial final P 1 V = P 2 V • equal volumes of gas at same T and P contain the same PV 1 2 T = constant number of moles T T 1 2 • V and # moles of gas are directly proportional •Use for any problems involving changes in P, T or V • Molar volume of any gas at STP= 22.4 L STP = 0°C 1 atm problem 1 V A gas has a pressure of 0.454 atm at -15°C and a volume 1 = constant of 3.48 L. If the conditions are changed so that the n 1 temperature is 36°C and pressure is 0.616 atm, what will the new volume be? final conditions Initial conditions V = V P 1 = 0.454 atm P 2 = 0.616 atm 1 2 n 1 n 2 V 1 = 3.48 L V 2 = ? T 1 = -15°C + 273 = 258K T 2 = 36°C + 273 = 309K problem 2 A gas at 35°C and 1 atm has a volume of 0.675 L. With P 1 V = P 2 V 1 2 pressure constant, the temperature is lowered and the volume T T of the gas reduces to 535 ml. Calculate the final T. 1 2 final conditions Initial conditions P 1 = 1.0 atm P 2 = 1.0 atm 2 = V 1 � T 2 � P V 1 V 1 = 0.675 L V 2 = 535 ml = 0.535 L T 1 � P 2 T 1 = 35°C + 273 = 308K T 2 = ? P 1 V = P 2 V V = V 2 = V 2 � T Eliminate P 1 2 1 2 2 = 3.48L 3.48L � 309K 309K � 0.454 atm 1 T T T V V 2 = T T V 1 2 258K � 0.616 atm 258K 1 2 1 0.535 L 0.535 L � 308K � T 2 = K = 244 K V 2 = 3.07L 0.675 L 2
The Ideal Gas Law The Ideal Gas Law •Summarizes all the Gas Laws: Charles: V directly proportional T Boyle: V inversely proportional P V T v Absolute zero P Avogadro: V directly proportional n 3
Recommend
More recommend