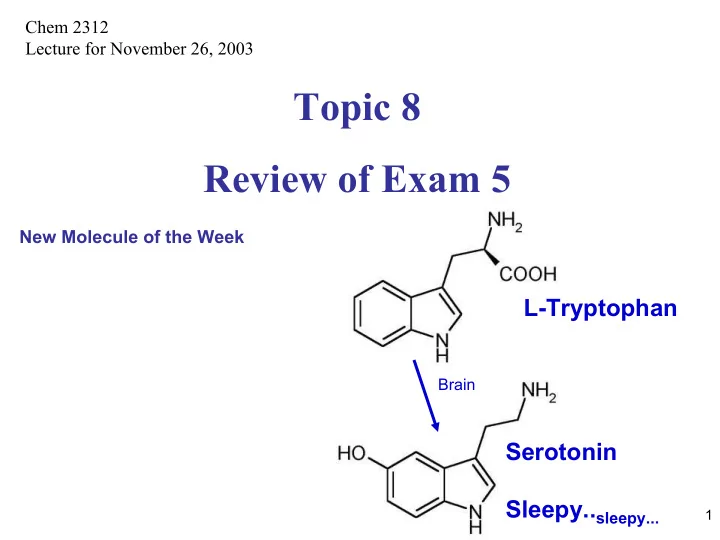

Chem 2312 Lecture for November 26, 2003 Topic 8 Review of Exam 5 New Molecule of the Week L-Tryptophan Brain Serotonin Sleepy.. sleepy... 1
1.Which structure below is 3-aminopropanoic acid ii) Which structure below is N,N -Dimethylcycloheptylamine iii) Which structure below is 4-chloro- N -ethyl-3-nitroaniline iv) Which structure below is 2,2-Dimethyl-1,3-propanediamine O O NH 2 O H O N O OH H OH NH 2 NH 2 C1 C2 C3 C4 NO 2 NH N H 2 N NH 2 H H N N N HN Cl C8 C9 C10 C5 C6 C7 NH 2 HN N Cl H 2 N N NO 2 H H 2 N NH 2 NO 2 Cl C14 C15 C11 C12 C13 None of the above 2 C16
Provide a reasonable reaction mechanism (include lone electron pairs, charges, and curved arrows for all atoms involved in the reaction) for the formation of product BB starting with A1 and A2. O O O OH O O + H H O O O O A1 A2 BB OH H O O + H 2 O A 1 A 2 BB O O H O O H H O O O O O H H O O H H O H H O O O O O O H 3 H H
Provide the structure of the major organic product(s) for 6 of the following 8 reactions If there are two or more major products show all of them. NaOCH 3 /CH 3 OH O a O H 3 O + O O O 1. NaOEt/EtOH, CH 3 I 2. NaOEt/EtOH, CH 3 CH 2 Br O O O b 3. H 2 O, NaOH 4. H 3 O + /heat O O O 1. c Cl O N 2. H 3 O + 1. LDA d O d O 2. CH 3 -CH 2 -Br O O 4
Provide the structure of the major organic product(s) for 6 of the following 8 reactions If there are two or more major products show all of them. 1. KOH O 2. CH 3 -CH 2 -CH 2 -Br e. NH H 2 N 3. H 2 N-NH 2 O O f. 1. HNEt 2 (excess) 2. LiAlH 4 Cl N 3. H 2 O 1. NaN 3 g. 2. LiAlH 4 Br NH 2 3. H 2 O 1. Heat + HO - h. N 5
Provide a synthetic pathway for DD starting with CC as shown below. Develop your synthetic scheme so as to maximize formation of DD with as few steps as possible. Describe all reagents, reaction conditions needed, and intermediate chemical structures formed due to proposed chemical reactions O O NH 2 NH 2 NH 2 NH 2 DD CC DD CC Cl AlCl 3 O O Br N NH 2 NaOH, Br 2 H NH 2 6
REMEMBER>>>>Problem from Solomoms and Fryhle 15.34 Br O FeBr 3 /Br 2 Why Not other Ring?? O Br O O O O + Which Ring Will Be Attacked? Br 15.35 FeBr 3 /Br 2 O Br O O + Which Ring Will Be Attacked? Br FeBr 3 /Br 2 NH O H H Br N N O O + Which Ring Will Be Attacked? O Br O FeBr 3 /Br 2 O O Symmetrical Which Ring Will Be Attacked? 7
Identify the reagent(s) and conditions required to transform the following starting materials into the corresponding products for the reactions described below. H O 1 H 2 N-Et (excess) N i LAH or H 2 /Pt 2 ii O NH 2 1 SOCl 2 OH 2 KN 3 3 Heat 4 H 2 O 8
Identify the reagent(s) and conditions required to transform the following starting materials into the corresponding products for the reactions described below. iii O O O 1 NaOEt/EtOH 2 H-C(O)-OEt H 2 O 3 9
Determine the order of BASICITY for the following aniline derivatives shown below, please place answer in the adjacent box. NH 2 NH 2 NH 2 NO 2 CH 3 NO 2 CH 3 Cl A1 A2 A3 Answer I: A1(most basic) > A2 > A3 (least basic) Answer II: A3(most basic) > A2 > A1 (least basic) Answer III: A1 (most basic) > A3 > A2 (least basic) Answer IV: A3 (most basic) > A1 > A2 (least basic) Answer V: A2 (most basic) > A1 > A3 (least basic) Answer VI: A2 (most basic) > A3 > A1 (least basic) Answer VII: none of the above Please justify your selection of the Most basic and Least Basic compound for 6a: In general, the more electron density on the N of aniline the stronger a base, for A1 their are 2 electron donating groups on the aromatic ring which will put greater electron density on N; for A3 this aniline derivative has 2 electron withdrawing groups (Note: electron with drawing ability of NO 2 >> Cl) that remove electron density from the N thereby reducing its electron density/basicity 10
Determine the order of BASICITY for the following nitrogen compounds shown below, please place answer in the adjacent box (4 marks). Answer XI: B1(most basic) > B2 > B3 (least basic) Answer XII: B3(most basic) > B2 > B1 (least basic) Answer XIII: B1 (most basic) > B3 > B2 (least basic) Answer XIV: B3 (most basic) > B1 > B2 (least basic) Answer XV: B2 (most basic) > B1 > B3 (least basic) Answer XVI: B2 (most basic) > B3 > B1 (least basic) Answer XVII: none of the above O NH 3 NH 2 NH 2 B1 B2 B3 What is the hybridization of the N in B1:____sp 3 _______ What is the hybridization of the N in B2:____sp 2 _______ sp 2 hybridized nitrogen atom is found in an amide functional group. 11
Please Remember All Exam re-grades need be handed within one week Please let your students know that the *three-week period* for them to complete their Course/Instructor Opinion Surveys about their classes and their professors begins this coming Monday. COURSE SURVEYS: From midnight, Monday, November 24-midnight, Friday, December 12--Course Surveys will be online, 24/7 (EXCEPT on Tuesdays, Thursdays, and Saturdays from midnight-3AM--the system will be DOWN for maintenance at those times). Students: To access the surveys for each of your classes: www.coursesurvey.gatech.edu/student_login.cfm 12
Please Remember Fall 2003 Finals Schedule http://www.oscarweb.gatech.edu/fall/oscar/ finals.html Final exams are given in the lecture hall that you used during the semester. Class MWF 1:00>> Exam Period 4 Exam Date TUES:12/9 8:00-10:50 am 13
Recommend
More recommend