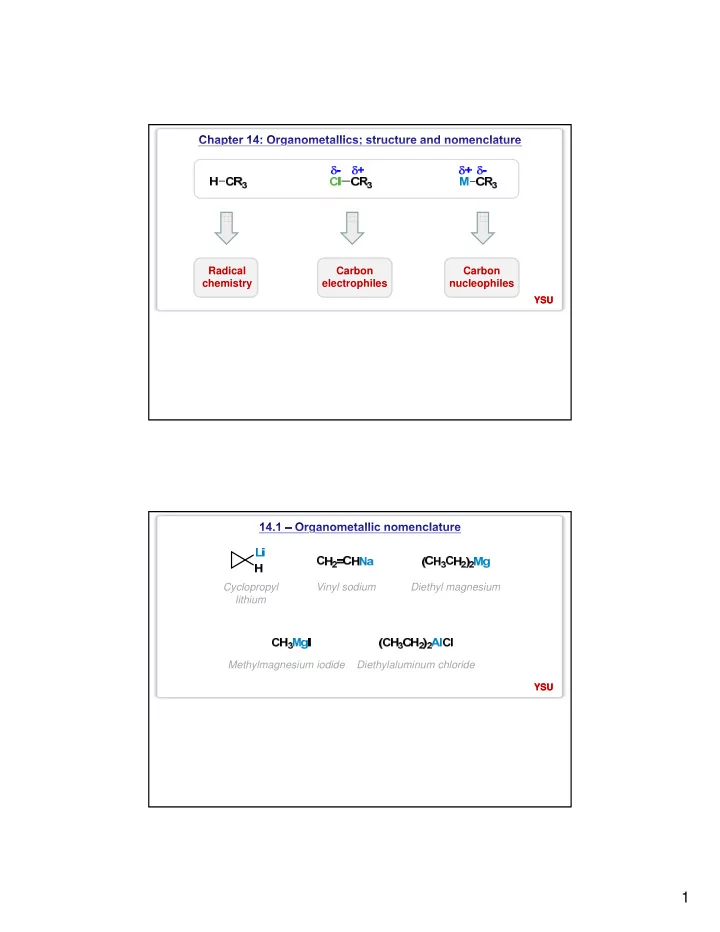

Radical Carbon Carbon chemistry electrophiles nucleophiles YSU YSU Cyclopropyl Vinyl sodium Diethyl magnesium lithium Methylmagnesium iodide Diethylaluminum chloride YSU YSU 1
YSU YSU Organolithiums YSU YSU 2
Organomagnesium compounds – Grignard reagents YSU YSU CH 3 Li, (CH 3 ) 3 CLi, n ‐ BuLi are extremely powerful bases Convenient preparation of Lithium Diisopropylamide (LDA) YSU YSU 3
Lab Experiment Grignard Reagent Preparation • Glassware must be clean and dry • Dry with the “heat gun” • Cool then begin experiment Be careful to use the right ether! YSU YSU YSU YSU 4
Carbonyl polarization: Grignard polarization: YSU YSU Carbonyl polarization: Grignard polarization: YSU YSU 5
New Mechanism: Nucleophilic Addition Most often followed by a quench with acid: YSU YSU Overall Sequence: Nucleophilic Addition then quench Very versatile alcohol synthesis Works well with most aldehydes & ketones YSU YSU 6
Example: Starting material Product 7 6 5 4 3 2 1 0 7 6 5 4 3 2 1 0 PPM PPM YSU YSU Cl OH Example: 1. Mg, ether Product IR: 3200 cm ‐ 1 2. CH 3 CHO 3. H 3 O + Starting material Starting material Product Product 70 60 50 40 30 20 10 0 80 70 60 50 40 30 20 10 0 PPM PPM YSU YSU 7
Product MS: M + = 254 Starting material S i i l P Product d 3 2 1 0 7 6 5 4 3 2 1 0 PPM PPM YSU YSU 1. NaNH 2 H 3 C C C H H 3 C C C CH 2 OH 2. H 2 C=O 3. H 3 O + H 3 C H C O C OH H 3 C C C Li C 1. 2. H 3 O + YSU YSU 8
Target Precursor molecule molecules (synthetic equivalent) YSU YSU 2 ‐ deoxy ‐ D ‐ ribose D ‐ mannopyranose YSU YSU 9
(synthetic equivalent) YSU YSU Example: Either will work on paper (i.e. on exams) YSU YSU 10
OH MgBr Example: O + H O + BrMg H YSU YSU H O OH MgBr Br + OH 1. Mg, THF Br 2. H O YSU YSU 3. H 3 O + 11
YSU YSU O OH Retrosynthesis: H 3 CO 2 CH 3 MgBr + OH 1. Mg, THF Synthesis: Synthesis: CH B CH 3 Br H 3 C 2. PhCO 2 CH 3 (0.5 eq) H 3 C 3. H 3 O + OH MgBr O Also possible : + YSU YSU 12
Will cover 14.11 in Special Topics at end of semester YSU YSU Iodomethyl zinc iodide Simmons ‐ Smith reaction YSU YSU 13
Will cover sections 14.13 – 14.17 in Special Topics at end of semester YSU YSU 14
Recommend
More recommend